Biocompatible glyconanomaterials based on HPMA-copolymer for specific targeting of galectin-3
- PMID: 30236114
- PMCID: PMC6146777
- DOI: 10.1186/s12951-018-0399-1
Biocompatible glyconanomaterials based on HPMA-copolymer for specific targeting of galectin-3
Abstract
Background: Galectin-3 (Gal-3) is a promising target in cancer therapy with a high therapeutic potential due to its abundant localization within the tumor tissue and its involvement in tumor development and proliferation. Potential clinical application of Gal-3-targeted inhibitors is often complicated by their insufficient selectivity or low biocompatibility. Nanomaterials based on N-(2-hydroxypropyl)methacrylamide (HPMA) nanocarrier are attractive for in vivo application due to their good water solubility and lack of toxicity and immunogenicity. Their conjugation with tailored carbohydrate ligands can yield specific glyconanomaterials applicable for targeting biomedicinally relevant lectins like Gal-3.
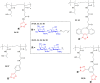
Results: In the present study we describe the synthesis and the structure-affinity relationship study of novel Gal-3-targeted glyconanomaterials, based on hydrophilic HPMA nanocarriers. HPMA nanocarriers decorated with varying amounts of Gal-3 specific epitope GalNAcβ1,4GlcNAc (LacdiNAc) were analyzed in a competitive ELISA-type assay and their binding kinetics was described by surface plasmon resonance. We showed the impact of various linker types and epitope distribution on the binding affinity to Gal-3. The synthesis of specific functionalized LacdiNAc epitopes was accomplished under the catalysis by mutant β-N-acetylhexosaminidases. The glycans were conjugated to statistic HPMA copolymer precursors through diverse linkers in a defined pattern and density using Cu(I)-catalyzed azide-alkyne cycloaddition. The resulting water-soluble and structurally flexible synthetic glyconanomaterials exhibited affinity to Gal-3 in low μM range.
Conclusions: The results of this study reveal the relation between the linker structure, glycan distribution and the affinity of the glycopolymer nanomaterial to Gal-3. They pave the way to specific biomedicinal glyconanomaterials that target Gal-3 as a therapeutic goal in cancerogenesis and other disorders.
Keywords: Carbohydrate; ELISA; Galectin-3; Glyconanomaterial; HPMA copolymer; Surface plasmon resonance.
Figures




Similar articles
-
High-Affinity N-(2-Hydroxypropyl)methacrylamide Copolymers with Tailored N-Acetyllactosamine Presentation Discriminate between Galectins.Biomacromolecules. 2020 Feb 10;21(2):641-652. doi: 10.1021/acs.biomac.9b01370. Epub 2020 Jan 23. Biomacromolecules. 2020. PMID: 31904940
-
Treatment of prostate carcinoma with (galectin-3)-targeted HPMA copolymer-(G3-C12)-5-Fluorouracil conjugates.Biomaterials. 2012 Mar;33(7):2260-71. doi: 10.1016/j.biomaterials.2011.12.007. Epub 2011 Dec 19. Biomaterials. 2012. PMID: 22189143
-
Tailored Multivalent Neo-Glycoproteins: Synthesis, Evaluation, and Application of a Library of Galectin-3-Binding Glycan Ligands.Bioconjug Chem. 2017 Nov 15;28(11):2832-2840. doi: 10.1021/acs.bioconjchem.7b00520. Epub 2017 Oct 18. Bioconjug Chem. 2017. PMID: 28976746
-
HPMA Copolymers: A Versatile Platform for Targeted Peptide Drug Delivery.Biomolecules. 2025 Apr 17;15(4):596. doi: 10.3390/biom15040596. Biomolecules. 2025. PMID: 40305357 Free PMC article. Review.
-
Synthetic glycoconjugates inhibitors of tumor-related galectin-3: an update.Glycoconj J. 2016 Dec;33(6):853-876. doi: 10.1007/s10719-016-9721-z. Epub 2016 Aug 15. Glycoconj J. 2016. PMID: 27526114 Review.
Cited by
-
Acceptor Specificity of β-N-Acetylhexosaminidase from Talaromyces flavus: A Rational Explanation.Int J Mol Sci. 2019 Dec 7;20(24):6181. doi: 10.3390/ijms20246181. Int J Mol Sci. 2019. PMID: 31817903 Free PMC article.
-
Nanoodor Particles Deliver Drugs to Central Nervous System via Olfactory Pathway.Adv Sci (Weinh). 2025 Apr;12(15):e2408908. doi: 10.1002/advs.202408908. Epub 2025 Feb 25. Adv Sci (Weinh). 2025. PMID: 39998409 Free PMC article.
-
Targeting Galectins With Glycomimetics.Front Chem. 2020 Aug 7;8:593. doi: 10.3389/fchem.2020.00593. eCollection 2020. Front Chem. 2020. PMID: 32850631 Free PMC article. Review.
-
Exploring multivalent carbohydrate-protein interactions by NMR.Chem Soc Rev. 2023 Mar 6;52(5):1591-1613. doi: 10.1039/d2cs00983h. Chem Soc Rev. 2023. PMID: 36753338 Free PMC article. Review.
-
Mutation Hotspot for Changing the Substrate Specificity of β-N-Acetylhexosaminidase: A Library of GlcNAcases.Int J Mol Sci. 2022 Oct 18;23(20):12456. doi: 10.3390/ijms232012456. Int J Mol Sci. 2022. PMID: 36293310 Free PMC article.
References
-
- Lundquist JJ, Toone EJ. The cluster glycoside effect. Chem Rev. 2002;102(555–57):8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

