The marsupial trypanosome Trypanosoma copemani is not an obligate intracellular parasite, although it adversely affects cell health
- PMID: 30236162
- PMCID: PMC6148770
- DOI: 10.1186/s13071-018-3092-1
The marsupial trypanosome Trypanosoma copemani is not an obligate intracellular parasite, although it adversely affects cell health
Abstract
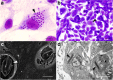
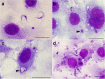
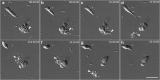
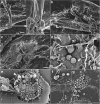
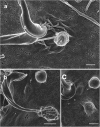
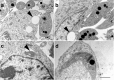
Background: Trypanosoma cruzi invades and replicates inside mammalian cells, which can lead to chronic Chagas disease in humans. Trypanosoma copemani infects Australian marsupials and recent investigations indicate it may be able to invade mammalian cells in vitro, similar to T. cruzi. Here, T. cruzi 10R26 strain (TcIIa) and two strains of T. copemani [genotype 1 (G1) and genotype 2 (G2)] were incubated with marsupial cells in vitro. Live-cell time-lapse and fluorescent microscopy, combined with high-resolution microscopy (transmission and scanning electron microscopy) were used to investigate surface interactions between parasites and mammalian cells.
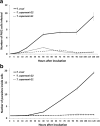
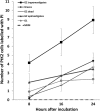
Results: The number of parasites invading cells was significantly higher in T. cruzi compared to either genotype of T. copemani, between which there was no significant difference. While capable of cellular invasion, T. copemani did not multiply in host cells in vitro as there was no increase in intracellular amastigotes over time and no release of new trypomastigotes from host cells, as observed in T. cruzi. Exposure of host cells to G2 trypomastigotes resulted in increased host cell membrane permeability within 24 h of infection, and host cell death/blebbing was also observed. G2 parasites also became embedded in the host cell membrane.
Conclusions: Trypanosoma copemani is unlikely to have an obligate intracellular life-cycle like T. cruzi. However, T. copemani adversely affects cell health in vitro and should be investigated in vivo in infected host tissues to better understand this host-parasite relationship. Future research should focus on increasing understanding of the T. copemani life history and the genetic, physiological and ecological differences between different genotypes.
Keywords: Australia; Host-parasite interactions; Marsupials; Trypanosoma copemani; Trypanosoma cruzi.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable. No animal tissues or human samples were used in this study and ethics approval was not required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- WHO. Chagas disease (American trypanosomiasis). World Health Organ Fact Sheet 340. 2015. http://www.who.int/mediacentre/factsheets/fs340/en/. Accessed 28 Jul 2018.
-
- Munoz-Saravia SG, Haberland A, Wallukat G, Schimke I. Chronic Chagas’ heart disease: a disease on its way to becoming a worldwide health problem: epidemiology, etiopathology, treatment, pathogenesis and laboratory medicine. Heart Fail Rev. 2010;17:45–64. doi: 10.1007/s10741-010-9211-5. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

