Retinoic acid signaling balances adult distal lung epithelial progenitor cell growth and differentiation
- PMID: 30236449
- PMCID: PMC6197151
- DOI: 10.1016/j.ebiom.2018.09.002
Retinoic acid signaling balances adult distal lung epithelial progenitor cell growth and differentiation
Abstract
Background: Despite compelling data describing pro-regenerative effects of all-trans retinoic acid (ATRA) in pre-clinical models of chronic obstructive pulmonary disease (COPD), clinical trials using retinoids for emphysema patients have failed. Crucial information about the specific role of RA signaling in adult rodent and human lung epithelial progenitor cells is largely missing.
Methods: Adult lung organoid cultures were generated from isolated primary mouse and human lung epithelial cells, and incubated with pharmacological pathway modulators and recombinant proteins. Organoid number and size were measured, and differentiation was assessed with quantitative immunofluorescence and gene expression analyses.
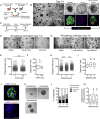
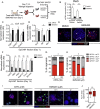
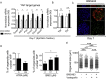
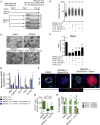
Findings: We unexpectedly found that ATRA decreased lung organoid size, whereas RA pathway inhibition increased mouse and human lung organoid size. RA pathway inhibition stimulated mouse lung epithelial proliferation via YAP pathway activation and epithelial-mesenchymal FGF signaling, while concomitantly suppressing alveolar and airway differentiation. HDAC inhibition rescued differentiation in growth-augmented lung organoids.
Interpretation: In contrast to prevailing notions, our study suggests that regenerative pharmacology using transient RA pathway inhibition followed by HDAC inhibition might hold promise to promote lung epithelial regeneration in diseased adult lung tissue. FUND: This project is funded by the Lung Foundation Netherlands (Longfonds) grant 6.1.14.009 (RG, MK, JS, PSH) and W2/W3 Professorship Award by the Helmholtz Association, Berlin, Germany (MK).
Keywords: Adult stem cells; COPD/emphysema; Lung epithelial organoids; Lung repair/regeneration; Retinoic acid.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Rabe K.F., Watz H. Chronic obstructive pulmonary disease. Lancet. 2017 May 13;389(10082):1931–1940. [PubMed PMID: 28513453] - PubMed
-
- Vogelmeier C.F., Criner G.J., Martinez F.J., Anzueto A., Barnes P.J., Bourbeau J. Global Strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017 Mar 1;195(5):557–582. [PubMed PMID: 28128970] - PubMed
-
- Hogan B.L., Barkauskas C.E., Chapman H.A., Epstein J.A., Jain R., Hsia C.C. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014 Aug 07;15(2):123–138. [PubMed PMID: 25105578. Pubmed Central PMCID: 4212493] - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

