Development of the human female reproductive tract
- PMID: 30236463
- PMCID: PMC6234064
- DOI: 10.1016/j.diff.2018.09.001
Development of the human female reproductive tract
Abstract
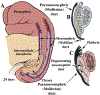
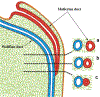
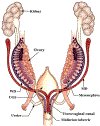
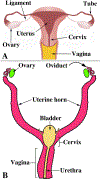
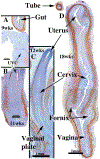
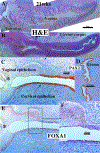
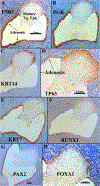
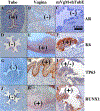
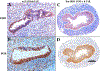
Development of the human female reproductive tract is reviewed from the ambisexual stage to advanced development of the uterine tube, uterine corpus, uterine cervix and vagina at 22 weeks. Historically this topic has been under-represented in the literature, and for the most part is based upon hematoxylin and eosin stained sections. Recent immunohistochemical studies for PAX2 (reactive with Müllerian epithelium) and FOXA1 (reactive with urogenital sinus epithelium and its known pelvic derivatives) shed light on an age-old debate on the derivation of vaginal epithelium supporting the idea that human vaginal epithelium derives solely from urogenital sinus epithelium. Aside for the vagina, most of the female reproductive tract is derived from the Müllerian ducts, which fuse in the midline to form the uterovaginal canal, the precursor of uterine corpus and uterine cervix an important player in vaginal development as well. Epithelial and mesenchymal differentiation markers are described during human female reproductive tract development (keratins, homeobox proteins (HOXA11 and ISL1), steroid receptors (estrogen receptor alpha and progesterone receptor), transcription factors and signaling molecules (TP63 and RUNX1), which are expressed in a temporally and spatially dynamic fashion. The utility of xenografts and epithelial-mesenchymal tissue recombination studies are reviewed.
Keywords: Cervix; Human Müllerian duct; Urogenital sinus; Uterovaginal canal; Uterus; Vagina; Wolffian duct.
Copyright © 2018 International Society of Differentiation. Published by Elsevier B.V. All rights reserved.
Figures
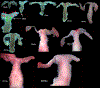







References
-
- Aboseif S, El-Sakka A, Young P, and Cunha G (1999) Mesenchymal reprogramming of adult human epithelial differentiation. Differentiation; research in biological diversity 65:113–118. - PubMed
-
- Baskin L, DiSandro M, Li Y, Li W, Hayward S, and Cunha G (2001) Mesenchymal-epithelial interactions in bladder smooth muscle development: effects of the local tissue environment. The Journal of urology 165:1283–1288. - PubMed
-
- Baskin LS, Hayward SW, Young P, and Cunha GR (1996) Ontogeny of the rat bladder: Smooth muscle and epithelial differentiation. Acta anatomica 155:104112. - PubMed
-
- Bern HA, Mills KT, Ostrander PI, Schoenrock B, Graveline B, and Plapinger L (1984) Cervicovaginal abnormalities in BALB/c mice treated neonatally with sex hormones. Teratology 30:267–274. - PubMed
-
- Bern HA, and Talamantes FJ (1981) Neonatal mouse models and their relation to disease in the humal female In: Herbst A, and Bern HA (eds) Developmental Effects of Diethylstilbestrol (DES) in Pregnancy Thieme Stratton Inc., New York, pp. 129–147.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

