Epigenetics and Epigenomics: Implications for Diabetes and Obesity
- PMID: 30237160
- PMCID: PMC6463748
- DOI: 10.2337/db18-0537
Epigenetics and Epigenomics: Implications for Diabetes and Obesity
Abstract
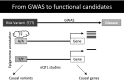
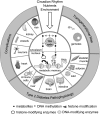
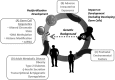
The American Diabetes Association convened a research symposium, "Epigenetics and Epigenomics: Implications for Diabetes and Obesity" on 17-19 November 2017. International experts in genetics, epigenetics, computational biology, and physiology discussed the current state of understanding of the relationships between genetics, epigenetics, and environment in diabetes and examined existing evidence for the role of epigenetic factors in regulating metabolism and the risk of diabetes and its complications. The authors summarize the presentations, which highlight how the complex interactions between genes and environment may in part be mediated through epigenetic changes and how information about nutritional and other environmental stimuli can be transmitted to the next generation. In addition, the authors present expert consensus on knowledge gaps and research recommendations for the field.
© 2018 by the American Diabetes Association.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

