Functional redundancy of frizzled 3 and frizzled 6 in planar cell polarity control of mouse hair follicles
- PMID: 30237242
- PMCID: PMC10682934
- DOI: 10.1242/dev.168468
Functional redundancy of frizzled 3 and frizzled 6 in planar cell polarity control of mouse hair follicles
Abstract
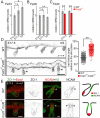
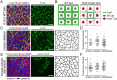
The orientation of mouse hair follicles is controlled by the planar cell polarity (PCP) pathway. Mutations in PCP genes result in two categories of hair mis-orientation phenotype: randomly oriented and vertically oriented to the skin surface. Here, we demonstrate that the randomly oriented hair phenotype observed in frizzled 6 (Fzd6) mutants results from a partial loss of the polarity, due to the functional redundancy of another closely related frizzled gene, Fzd3 Double knockout of Fzd3 and Fzd6 globally, or only in the skin, led to vertically oriented hair follicles and a total loss of anterior-posterior polarity. Furthermore, we provide evidence that, contrary to the prevailing model, asymmetrical localization of the Fzd6 protein is not observed in skin epithelial cells. Through transcriptome analyses and in vitro studies, we show collagen triple helix repeat containing 1 (Cthrc1) to be a potential downstream effector of Fzd6, but not of Fzd3. Cthrc1 binds directly to the extracellular domains of Fzd3 and Fzd6 to enhance the Wnt/PCP signaling. These results suggest that Fzd3 and Fzd6 play a redundant role in controlling the polarity of developing skin, but through non-identical mechanisms.
Keywords: Fzd3; Fzd6; Hair follicle; Mouse development; Planar cell polarity; Skin.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures





Similar articles
-
The spatio-temporal domains of Frizzled6 action in planar polarity control of hair follicle orientation.Dev Biol. 2016 Jan 1;409(1):181-193. doi: 10.1016/j.ydbio.2015.10.027. Epub 2015 Nov 10. Dev Biol. 2016. PMID: 26517967 Free PMC article.
-
Overactive Wnt5a signaling disrupts hair follicle polarity during mouse skin development.Development. 2022 Nov 15;149(22):dev200816. doi: 10.1242/dev.200816. Epub 2022 Nov 16. Development. 2022. PMID: 36305473 Free PMC article.
-
Frizzled3 and Frizzled6 Cooperate with Vangl2 to Direct Cochlear Innervation by Type II Spiral Ganglion Neurons.J Neurosci. 2019 Oct 9;39(41):8013-8023. doi: 10.1523/JNEUROSCI.1740-19.2019. Epub 2019 Aug 28. J Neurosci. 2019. PMID: 31462532 Free PMC article.
-
Frizzled Receptors in Development and Disease.Curr Top Dev Biol. 2016;117:113-39. doi: 10.1016/bs.ctdb.2015.11.028. Epub 2016 Jan 27. Curr Top Dev Biol. 2016. PMID: 26969975 Free PMC article. Review.
-
Wnt-Frizzled/planar cell polarity signaling: cellular orientation by facing the wind (Wnt).Annu Rev Cell Dev Biol. 2015;31:623-46. doi: 10.1146/annurev-cellbio-100814-125315. Annu Rev Cell Dev Biol. 2015. PMID: 26566118 Free PMC article. Review.
Cited by
-
Modeling Paracrine Noncanonical Wnt Signaling In Vitro.J Vis Exp. 2021 Dec 10;(178):10.3791/63247. doi: 10.3791/63247. J Vis Exp. 2021. PMID: 34958087 Free PMC article.
-
Frizzled receptors: gatekeepers of Wnt signaling in development and disease.Front Cell Dev Biol. 2025 May 1;13:1599355. doi: 10.3389/fcell.2025.1599355. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40376615 Free PMC article. Review.
-
Frizzled Receptors in Tumors, Focusing on Signaling, Roles, Modulation Mechanisms, and Targeted Therapies.Oncol Res. 2021 Mar 16;28(6):661-674. doi: 10.3727/096504020X16014648664459. Epub 2020 Sep 30. Oncol Res. 2021. PMID: 32998794 Free PMC article. Review.
-
New mouse models for high resolution and live imaging of planar cell polarity proteins in vivo.Development. 2021 Sep 15;148(18):dev199695. doi: 10.1242/dev.199695. Epub 2021 Sep 23. Development. 2021. PMID: 34463728 Free PMC article.
-
ARHGEF3 Regulates Hair Follicle Morphogenesis.bioRxiv [Preprint]. 2024 Sep 15:2024.09.13.612256. doi: 10.1101/2024.09.13.612256. bioRxiv. 2024. PMID: 39314354 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

