Hypersensitivity reactions to asparaginase in mice are mediated by anti-asparaginase IgE and IgG and the immunoglobulin receptors FcεRI and FcγRIII
- PMID: 30237274
- PMCID: PMC6355496
- DOI: 10.3324/haematol.2018.199448
Hypersensitivity reactions to asparaginase in mice are mediated by anti-asparaginase IgE and IgG and the immunoglobulin receptors FcεRI and FcγRIII
Abstract
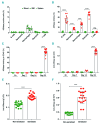
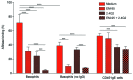
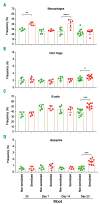
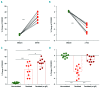
Asparaginase is an important drug for the treatment of leukemias. However, anti-asparaginase antibodies often develop, which can decrease asparaginase drug levels and increase the risk of relapse. The aim of this study is to identify the immunoglobulin isotypes and receptors responsible for asparaginase hypersensitivities. Mice immunized with asparaginase developed anti-asparaginase IgG1 and IgE antibodies, and challenging the sensitized mice with asparaginase induced severe hypersensitivity reactions. Flow cytometry analysis indicated that macrophages/monocytes, neutrophils, and basophils bind asparaginase ex vivo through FcγRIII. In contrast, asparaginase binding to basophils was dependent on FcγRIII and IgE. Consistent with the asparaginase binding data, basophil activation by asparaginase occurred via both IgG/FcγRIII and IgE/FcεRI. Depleting >95% of B cells suppressed IgG but not IgE-dependent hypersensitivity, while depleting CD4+ T cells provided complete protection. Combined treatment with either anti-IgE mAb plus a platelet-activating factor receptor antagonist or anti-FcγRIII mAb plus a H1 receptor antagonist suppressed asparaginase hypersensitivity. The observations indicate that asparaginase hypersensitivity is mediated by antigen-specific IgG and/or IgE through the immunoglobulin receptors FcγRIII and FcεRI, respectively. Provided that these results apply to humans, they emphasize the importance of monitoring both IgE- and IgG-mediated asparaginase hypersensitivities in patients receiving this agent.
Copyright © 2019 Ferrata Storti Foundation.
Figures


Similar articles
-
Asparaginase-specific basophil recognition and activation predict Asparaginase hypersensitivity in mice.Front Immunol. 2024 Apr 15;15:1392099. doi: 10.3389/fimmu.2024.1392099. eCollection 2024. Front Immunol. 2024. PMID: 38686384 Free PMC article.
-
Carboplatin-induced severe hypersensitivity reaction: role of IgE-dependent basophil activation and FcεRI.Cancer Sci. 2014 Nov;105(11):1472-9. doi: 10.1111/cas.12538. Epub 2014 Nov 5. Cancer Sci. 2014. PMID: 25230301 Free PMC article.
-
Asparaginase immune complexes induce Fc-γRIII-dependent hypersensitivity in naive mice.FASEB J. 2019 Oct;33(10):10996-11005. doi: 10.1096/fj.201900857. Epub 2019 Jul 5. FASEB J. 2019. PMID: 31284767 Free PMC article.
-
Human IgE-independent systemic anaphylaxis.J Allergy Clin Immunol. 2016 Jun;137(6):1674-1680. doi: 10.1016/j.jaci.2016.02.015. Epub 2016 Apr 26. J Allergy Clin Immunol. 2016. PMID: 27130857 Free PMC article. Review.
-
Anaphylaxis: lessons from mouse models.J Allergy Clin Immunol. 2007 Sep;120(3):506-15; quiz 516-7. doi: 10.1016/j.jaci.2007.07.033. J Allergy Clin Immunol. 2007. PMID: 17765751 Review.
Cited by
-
A Review of L-Asparaginase Hypersensitivity in Paediatric Acute Lymphoblastic Leukaemia Patients with Regard to the Measurement of Anti-Asparaginase Antibodies and Their Genetic Predisposition.Malays J Med Sci. 2023 Oct;30(5):40-51. doi: 10.21315/mjms2023.30.5.4. Epub 2023 Oct 30. Malays J Med Sci. 2023. PMID: 37928798 Free PMC article. Review.
-
Genetic inhibition of NFATC2 attenuates asparaginase hypersensitivity in mice.Blood Adv. 2020 Sep 22;4(18):4406-4416. doi: 10.1182/bloodadvances.2020002478. Blood Adv. 2020. PMID: 32931581 Free PMC article.
-
Case Report: Genetic Analysis of PEG-Asparaginase Induced Severe Hypertriglyceridemia in an Adult With Acute Lymphoblastic Leukaemia.Front Genet. 2022 Feb 14;13:832890. doi: 10.3389/fgene.2022.832890. eCollection 2022. Front Genet. 2022. PMID: 35237305 Free PMC article.
-
HLA alleles associated with asparaginase hypersensitivity in childhood ALL: a report from the DFCI Consortium.Pharmacogenomics. 2020 Jun;21(8):541-547. doi: 10.2217/pgs-2019-0195. Epub 2020 May 6. Pharmacogenomics. 2020. PMID: 32372697 Free PMC article.
-
Acute Myeloid Leukaemia and Acute Lymphoblastic Leukaemia Classification and Metabolic Characteristics for Informing and Advancing Treatment.Cancers (Basel). 2024 Dec 11;16(24):4136. doi: 10.3390/cancers16244136. Cancers (Basel). 2024. PMID: 39766036 Free PMC article. Review.
References
-
- Asselin BL, Ryan D, Frantz CN, et al. In vitro and in vivo killing of acute lymphoblastic leukemia cells by L-asparaginase. Cancer Res. 1989;49(15):4363–4368. - PubMed
-
- Oettgen HF, Stephenson PA, Schwartz MK, et al. Toxicity of E. coli L-asparaginase in man. Cancer. 1970;25(2):253–278. - PubMed
-
- Panosyan EH, Seibel NL, Martin-Aragon S, et al. Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: Children’s Cancer Group Study CCG-1961. J Pediatr Hematol Oncol. 2004;26(4):217–226. - PubMed
-
- Avramis VI, Sencer S, Periclou AP, et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children’s Cancer Group study. Blood. 2002;99(6):1986–1994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

