Synchronized mechanical oscillations at the cell-matrix interface in the formation of tensile tissue
- PMID: 30237286
- PMCID: PMC6176648
- DOI: 10.1073/pnas.1801759115
Synchronized mechanical oscillations at the cell-matrix interface in the formation of tensile tissue
Abstract
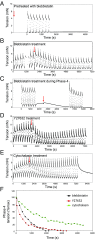
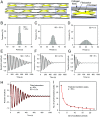
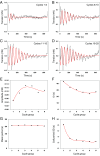
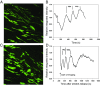
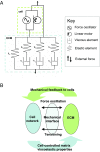
The formation of uniaxial fibrous tissues with defined viscoelastic properties implies the existence of an orchestrated mechanical interaction between the cytoskeleton and the extracellular matrix. This study addresses the nature of this interaction. The hypothesis is that this mechanical interplay underpins the mechanical development of the tissue. In embryonic tendon tissue, an early event in the development of a mechanically robust tissue is the interaction of the pointed tips of extracellular collagen fibrils with the fibroblast plasma membrane to form stable interface structures (fibripositors). Here, we used a fibroblast-generated tissue that is structurally and mechanically matched to embryonic tendon to demonstrate homeostasis of cell-derived and external strain-derived tension over repeated cycles of strain and relaxation. A cell-derived oscillatory tension component is evident in this matrix construct. This oscillatory tension involves synchronization of individual cell forces across the construct and is induced in each strain cycle by transient relaxation and transient tensioning of the tissue. The cell-derived tension along with the oscillatory component is absent in the presence of blebbistatin, which disrupts actinomyosin force generation of the cell. The time period of this oscillation (60-90 s) is well-defined in each tissue sample and matches a primary viscoelastic relaxation time. We hypothesize that this mechanical oscillation of fibroblasts with plasma membrane anchored collagen fibrils is a key factor in mechanical sensing and feedback regulation in the formation of tensile tissues.
Keywords: cell force; collagen; electron microscopy; fibroblast; tension.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. - PubMed
-
- Cameron AR, Frith JE, Cooper-White JJ. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials. 2011;32:5979–5993. - PubMed
-
- Cameron AR, Frith JE, Gomez GA, Yap AS, Cooper-White JJ. The effect of time-dependent deformation of viscoelastic hydrogels on myogenic induction and Rac1 activity in mesenchymal stem cells. Biomaterials. 2014;35:1857–1868. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

