The endothelial glycocalyx anchors von Willebrand factor fibers to the vascular endothelium
- PMID: 30237293
- PMCID: PMC6156889
- DOI: 10.1182/bloodadvances.2017013995
The endothelial glycocalyx anchors von Willebrand factor fibers to the vascular endothelium
Abstract
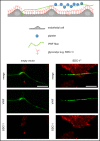
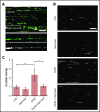
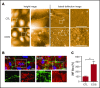
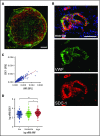
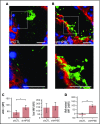
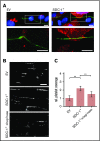
The dynamic change from a globular conformation to an elongated fiber determines the ability of von Willebrand factor (VWF) to trap platelets. Fiber formation is favored by the anchorage of VWF to the endothelial cell surface, and VWF-platelet aggregates on the endothelium contribute to inflammation, infection, and tumor progression. Although P-selectin and ανβ3-integrins may bind VWF, their precise role is unclear, and additional binding partners have been proposed. In the present study, we evaluated whether the endothelial glycocalyx anchors VWF fibers to the endothelium. Using microfluidic experiments, we showed that stabilization of the endothelial glycocalyx by chitosan oligosaccharides or overexpression of syndecan-1 (SDC-1) significantly supports the binding of VWF fibers to endothelial cells. Heparinase-mediated degradation or impaired synthesis of heparan sulfate (HS), a major component of the endothelial glycocalyx, reduces VWF fiber-dependent platelet recruitment. Molecular interaction studies using flow cytometry and live-cell fluorescence microscopy provided further evidence that VWF binds to HS linked to SDC-1. In a murine melanoma model, we found that protection of the endothelial glycocalyx through the silencing of heparanase increases the number of VWF fibers attached to the wall of tumor blood vessels. In conclusion, we identified HS chains as a relevant binding factor for VWF fibers at the endothelial cell surface in vitro and in vivo.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


References
-
- Huck V, Schneider MF, Gorzelanny C, Schneider SW. The various states of von Willebrand factor and their function in physiology and pathophysiology. Thromb Haemost. 2014;111(4):598-609. - PubMed
-
- Petri B, Broermann A, Li H, et al. von Willebrand factor promotes leukocyte extravasation. Blood. 2010;116(22):4712-4719. - PubMed
-
- Pappelbaum KI, Gorzelanny C, Grässle S, et al. Ultralarge von Willebrand factor fibers mediate luminal Staphylococcus aureus adhesion to an intact endothelial cell layer under shear stress. Circulation. 2013;128(1):50-59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

