Targeting TAZ-Driven Human Breast Cancer by Inhibiting a SKP2-p27 Signaling Axis
- PMID: 30237296
- PMCID: PMC6318031
- DOI: 10.1158/1541-7786.MCR-18-0332
Targeting TAZ-Driven Human Breast Cancer by Inhibiting a SKP2-p27 Signaling Axis
Abstract
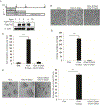
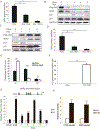
Deregulated expression of the transcriptional coactivator with PDZ-binding motif (WWTR1/TAZ) is a common feature of basal-like breast cancer (BLBC). Yet, how oncogenic TAZ regulates cell-cycle progression and proliferation in breast cancer remains poorly understood, and whether TAZ is required for tumor maintenance has not been established. Here, using an integrative oncogenomic approach, TAZ-dependent cellular programs essential for tumor growth and progression were identified. Significantly, TAZ-driven tumor cells required sustained TAZ expression, given that its withdrawal impaired both genesis and maintenance of solid tumors. Moreover, temporal inhibition of TAZ diminished the metastatic burden in established macroscopic pulmonary metastases. Mechanistic investigation revealed that TAZ controls distinct gene profiles that determine cancer cell fate through cell-cycle networks, including a specific, causal role for S-phase kinase-associated protein 2 (SKP2) in mediating the neoplastic state. Together, this study elucidates the molecular events that underpin the role of TAZ in BLBC and link to SKP2, a convergent communication node for multiple cancer signaling pathways, as a key downstream effector molecule. IMPLICATIONS: Understanding the molecular role of TAZ and its link to SKP2, a signaling convergent point and key regulator in BLBC, represents an important step toward the identification of novel therapeutic targets for TAZ-dependent breast cancer.
©2018 American Association for Cancer Research.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures



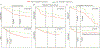
References
-
- Beca F, Polyak K. Intratumor Heterogeneity in Breast Cancer. Adv Exp Med Biol. 2016;882:169–89. - PubMed
-
- Paquet ER, Hallett MT. Absolute assignment of breast cancer intrinsic molecular subtype. J Natl Cancer Inst. 2015;107:357. - PubMed
-
- Holm J, Eriksson L, Ploner A et al. Assessment of Breast Cancer Risk Factors Reveals Subtype Heterogeneity. Cancer Res. 2017;77:3708–17. - PubMed
-
- Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: a critical review. J Clin Oncol. 2008;26:2568–81. - PubMed
-
- Dey N, Smith BR, Leyland-Jones B. Targeting basal-like breast cancers. Curr Drug Targets. 2012;13:1510–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

