A new era for understanding amyloid structures and disease
- PMID: 30237470
- PMCID: PMC7617691
- DOI: 10.1038/s41580-018-0060-8
A new era for understanding amyloid structures and disease
Abstract
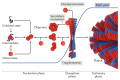
The aggregation of proteins into amyloid fibrils and their deposition into plaques and intracellular inclusions is the hallmark of amyloid disease. The accumulation and deposition of amyloid fibrils, collectively known as amyloidosis, is associated with many pathological conditions that can be associated with ageing, such as Alzheimer disease, Parkinson disease, type II diabetes and dialysis-related amyloidosis. However, elucidation of the atomic structure of amyloid fibrils formed from their intact protein precursors and how fibril formation relates to disease has remained elusive. Recent advances in structural biology techniques, including cryo-electron microscopy and solid-state NMR spectroscopy, have finally broken this impasse. The first near-atomic-resolution structures of amyloid fibrils formed in vitro, seeded from plaque material and analysed directly ex vivo are now available. The results reveal cross-β structures that are far more intricate than anticipated. Here, we describe these structures, highlighting their similarities and differences, and the basis for their toxicity. We discuss how amyloid structure may affect the ability of fibrils to spread to different sites in the cell and between organisms in a prion-like manner, along with their roles in disease. These molecular insights will aid in understanding the development and spread of amyloid diseases and are inspiring new strategies for therapeutic intervention.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Sipe JD, Cohen AS. Review: History of the amyloid fibril. J Struct Biol. 2000;130:88–98. - PubMed
-
- Goedert M, Wischik CM, Crowther RA, Walker JE, Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc Natl Acad Sci USA. 1988;85:4051–4055. doi: 10.1073/pnas.85.11.4051. - DOI - PMC - PubMed
-
- Protein Data Bank. Yearly growth of total structures. rcbs.org. 2018. http://www.rcsb.org/pdb/statistics/contentGrowthChart.do?content=total .
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

