Mesenchymal stem cells loaded with paclitaxel-poly(lactic- co-glycolic acid) nanoparticles for glioma-targeting therapy
- PMID: 30237710
- PMCID: PMC6136913
- DOI: 10.2147/IJN.S167142
Mesenchymal stem cells loaded with paclitaxel-poly(lactic- co-glycolic acid) nanoparticles for glioma-targeting therapy
Abstract
Background: Mesenchymal stem cells (MSCs) possess inherent tropism towards tumor cells, and so have attracted increased attention as targeted-therapy vehicles for glioma treatment.
Purpose: The objective of this study was to demonstrate the injection of MSCs loaded with paclitaxel (Ptx)-encapsulated poly(d,l-lactide-co-glycolide) (PLGA) nanoparticles (NPs) for orthotopic glioma therapy in rats.
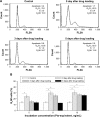
Methods: Ptx-PLGA NP-loaded MSC was obtained by incubating MSCs with Ptx-PLGA NPs. The drug transfer and cytotoxicity of Ptx-PLGA NP-loaded MSC against tumor cells were investigated in the transwell system. Biodistribution and antitumor activity was evaluated in the orthotopic glioma rats after contralateral injection.
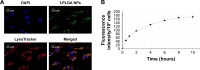
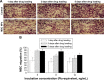
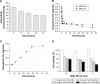
Results: The optimal dose of MSC-loaded Ptx-PLGA NPs (1 pg/cell Ptx) had little effect on MSC-migration capacity, cell cycle, or multilineage-differentiation potential. Compared with Ptx-primed MSCs, Ptx-PLGA NP-primed MSCs had enhanced sustained Ptx release in the form of free Ptx and Ptx NPs. Ptx transfer from MSCs to glioma cells could induce tumor cell death in vitro. As for distribution in vivo, NP-loaded fluorescent MSCs were tracked throughout the tumor mass for 2 days after therapeutic injection. Survival was significantly longer after contralateral implantation of Ptx-PLGA NP-loaded MSCs than those injected with Ptx-primed MSCs or Ptx-PLGA NPs alone.
Conclusion: Based on timing and sufficient Ptx transfer from the MSCs to the tumor cells, Ptx-PLGA NP-loaded MSC is effective for glioma treatment. Incorporation of chemotherapeutic drug-loaded NPs into MSCs is a promising strategy for tumor-targeted therapy.
Keywords: BMSCs; C6 cells; contralateral injection; drug targeting; orthotopic glioma.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures





Similar articles
-
Folate-receptor-targeted laser-activable poly(lactide-co-glycolic acid) nanoparticles loaded with paclitaxel/indocyanine green for photoacoustic/ultrasound imaging and chemo/photothermal therapy.Int J Nanomedicine. 2018 Sep 6;13:5139-5158. doi: 10.2147/IJN.S167043. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30233177 Free PMC article.
-
Synthesis, characterization, and evaluation of paclitaxel loaded in six-arm star-shaped poly(lactic-co-glycolic acid).Int J Nanomedicine. 2013;8:4315-26. doi: 10.2147/IJN.S51629. Epub 2013 Nov 7. Int J Nanomedicine. 2013. PMID: 24235829 Free PMC article.
-
Magnetic targeting of paclitaxel-loaded poly(lactic-co-glycolic acid)-based nanoparticles for the treatment of glioblastoma.Int J Nanomedicine. 2018 Aug 8;13:4509-4521. doi: 10.2147/IJN.S165184. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30127603 Free PMC article.
-
Fabrication and Use of Poly(d,l-lactide-co-glycolide)-Based Formulations Designed for Modified Release of 5-Fluorouracil.J Pharm Sci. 2018 Feb;107(2):513-528. doi: 10.1016/j.xphs.2017.10.012. Epub 2017 Oct 16. J Pharm Sci. 2018. PMID: 29045885 Free PMC article. Review.
-
Biomedical applications of PLGA nanoparticles in nanomedicine: advances in drug delivery systems and cancer therapy.Expert Opin Drug Deliv. 2023 Jul-Dec;20(7):937-954. doi: 10.1080/17425247.2023.2223941. Epub 2023 Jun 23. Expert Opin Drug Deliv. 2023. PMID: 37294853 Review.
Cited by
-
Similarities between Tumour Immune Response and Chronic Wound Microenvironment: Influence of Mesenchymal Stromal/Stem Cells.J Immunol Res. 2021 Mar 29;2021:6649314. doi: 10.1155/2021/6649314. eCollection 2021. J Immunol Res. 2021. PMID: 33860061 Free PMC article. Review.
-
Incorporation of paclitaxel in mesenchymal stem cells using nanoengineering upregulates antioxidant response, CXCR4 expression and enhances tumor homing.Mater Today Bio. 2023 Jan 30;19:100567. doi: 10.1016/j.mtbio.2023.100567. eCollection 2023 Apr. Mater Today Bio. 2023. PMID: 36747581 Free PMC article.
-
Modified mesenchymal stem cells in cancer therapy: A smart weapon requiring upgrades for wider clinical applications.World J Stem Cells. 2022 Jan 26;14(1):54-75. doi: 10.4252/wjsc.v14.i1.54. World J Stem Cells. 2022. PMID: 35126828 Free PMC article. Review.
-
Cell-Based Therapy for the Treatment of Glioblastoma: An Update from Preclinical to Clinical Studies.Cells. 2021 Dec 30;11(1):116. doi: 10.3390/cells11010116. Cells. 2021. PMID: 35011678 Free PMC article. Review.
-
Biomimetic cell membrane-coated poly(lactic-co-glycolic acid) nanoparticles for biomedical applications.Bioeng Transl Med. 2022 Nov 2;8(2):e10441. doi: 10.1002/btm2.10441. eCollection 2023 Mar. Bioeng Transl Med. 2022. PMID: 36925703 Free PMC article. Review.
References
-
- Zhang TY, Huang B, Yuan ZY, et al. Gene recombinant bone marrow mesenchymal stem cells as a tumor-targeted suicide gene delivery vehicle in pulmonary metastasis therapy using non-viral transfection. Nanomedicine. 2014;10(1):257–267. - PubMed
-
- Zhang TY, Huang B, Wu HB, et al. Synergistic effects of co-administration of suicide gene expressing mesenchymal stem cells and prodrug-encapsulated liposome on aggressive lung melanoma metastases in mice. J Control Release. 2015;209:260–271. - PubMed
-
- Choi SA, Lee YE, Kwak PA, et al. Clinically applicable human adipose tissue-derived mesenchymal stem cells delivering therapeutic genes to brainstem gliomas. Cancer Gene Ther. 2015;22(6):302–311. - PubMed
-
- Bexell D, Svensson A, Bengzon J. Stem cell-based therapy for malignant glioma. Cancer Treat Rev. 2013;39(4):358–365. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

