Constitutive Vagus Nerve Activation Modulates Immune Suppression in Sepsis Survivors
- PMID: 30237803
- PMCID: PMC6135874
- DOI: 10.3389/fimmu.2018.02032
Constitutive Vagus Nerve Activation Modulates Immune Suppression in Sepsis Survivors
Abstract
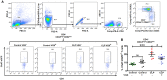
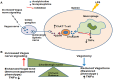
Patients surviving a septic episode exhibit persistent immune impairment and increased mortality due to enhanced vulnerability to infections. In the present study, using the cecal ligation and puncture (CLP) model of polymicrobial sepsis, we addressed the hypothesis that altered vagus nerve activity contributes to immune impairment in sepsis survivors. CLP-surviving mice exhibited less TNFα in serum following administration of LPS, a surrogate for an infectious challenge, than control-operated (control) mice. To evaluate the role of the vagus nerve in the diminished response to LPS, mice were subjected to bilateral subdiaphragmatic vagotomy at 2 weeks post-CLP. CLP-surviving vagotomized mice exhibited increased serum and tissue TNFα levels in response to LPS-challenge compared to CLP-surviving, non-vagotomized mice. Moreover, vagus nerve stimulation in control mice diminished the LPS-induced TNFα responses while having no effect in CLP mice, suggesting constitutive activation of vagus nerve signaling in CLP-survivors. The percentage of splenic CD4+ ChAT-EGFP+ T cells that relay vagus signals to macrophages was increased in CLP-survivors compared to control mice, and vagotomy in CLP-survivors resulted in a reduced percentage of ChAT-EGFP+ cells. Moreover, CD4 knockout CLP-surviving mice exhibited an enhanced LPS-induced TNFα response compared to wild-type mice, supporting a functional role for CD4+ ChAT+ T cells in mediating inhibition of LPS-induced TNFα responses in CLP-survivors. Blockade of the cholinergic anti-inflammatory pathway with methyllcaconitine, an α7 nicotinic acetylcholine receptor antagonist, restored LPS-induced TNFα responses in CLP-survivors. Our study demonstrates that the vagus nerve is constitutively active in CLP-survivors and contributes to the immune impairment.
Keywords: CD4+ ChAT+ T cell; TNFα; innate immune response; sepsis survivors; vagus tonic activity.
Figures



Similar articles
-
M1 cholinergic signaling in the brain modulates cytokine levels and splenic cell sub-phenotypes following cecal ligation and puncture.Mol Med. 2024 Feb 5;30(1):22. doi: 10.1186/s10020-024-00787-x. Mol Med. 2024. PMID: 38317082 Free PMC article.
-
[Influence of vagus nerve on multiple organ function and immune reaction of T lymphocytes in septic rats].Zhonghua Shao Shang Za Zhi. 2018 Nov 20;34(11):815-820. doi: 10.3760/cma.j.issn.1009-2587.2018.11.018. Zhonghua Shao Shang Za Zhi. 2018. PMID: 30481924 Chinese.
-
The Role of Acetylcholine in the Inflammatory Response in Animals Surviving Sepsis Induced by Cecal Ligation and Puncture.Mol Neurobiol. 2016 Dec;53(10):6635-6643. doi: 10.1007/s12035-015-9538-y. Epub 2015 Dec 5. Mol Neurobiol. 2016. PMID: 26637327
-
Expression and Function of the Cholinergic System in Immune Cells.Front Immunol. 2017 Sep 6;8:1085. doi: 10.3389/fimmu.2017.01085. eCollection 2017. Front Immunol. 2017. PMID: 28932225 Free PMC article. Review.
-
Manipulation of the inflammatory reflex as a therapeutic strategy.Cell Rep Med. 2022 Jul 19;3(7):100696. doi: 10.1016/j.xcrm.2022.100696. Cell Rep Med. 2022. PMID: 35858588 Free PMC article. Review.
Cited by
-
Immunomonitoring of Monocyte and Neutrophil Function in Critically Ill Patients: From Sepsis and/or Trauma to COVID-19.J Clin Med. 2021 Dec 12;10(24):5815. doi: 10.3390/jcm10245815. J Clin Med. 2021. PMID: 34945111 Free PMC article. Review.
-
Brain imaging and machine learning reveal uncoupled functional network for contextual threat memory in long sepsis.Sci Rep. 2024 Nov 12;14(1):27747. doi: 10.1038/s41598-024-79259-5. Sci Rep. 2024. PMID: 39533062 Free PMC article.
-
Vagus nerve stimulation modulates distinct acetylcholine receptors on B cells and limits the germinal center response.Sci Adv. 2024 Apr 26;10(17):eadn3760. doi: 10.1126/sciadv.adn3760. Epub 2024 Apr 26. Sci Adv. 2024. PMID: 38669336 Free PMC article.
-
The surviving sepsis campaign: basic/translational science research priorities.Intensive Care Med Exp. 2020 Jul 17;8(1):31. doi: 10.1186/s40635-020-00312-4. Intensive Care Med Exp. 2020. PMID: 32676795 Free PMC article.
-
Neuroimmune Regulation in Sepsis-Associated Encephalopathy: The Interaction Between the Brain and Peripheral Immunity.Front Neurol. 2022 Jun 27;13:892480. doi: 10.3389/fneur.2022.892480. eCollection 2022. Front Neurol. 2022. PMID: 35832175 Free PMC article. Review.
References
-
- Quartin AA, Schein RM, Kett DH, Peduzzi PN. Magnitude and duration of the effect of sepsis on survival. Department of veterans affairs systemic sepsis cooperative studies group. JAMA (1997) 277:1058–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

