Engineering xylose metabolism in thraustochytrid T18
- PMID: 30237825
- PMCID: PMC6139898
- DOI: 10.1186/s13068-018-1246-1
Engineering xylose metabolism in thraustochytrid T18
Abstract
Background: Thraustochytrids are heterotrophic, oleaginous, marine protists with a significant potential for biofuel production. High-value co-products can off-set production costs; however, the cost of raw materials, and in particular carbon, is a major challenge to developing an economical viable production process. The use of hemicellulosic carbon derived from agricultural waste, which is rich in xylose and glucose, has been proposed as a sustainable and low-cost approach. Thraustochytrid strain T18 is a commercialized environmental isolate that readily consumes glucose, attaining impressive biomass, and oil production levels. However, neither thraustochytrid growth capabilities in the presence of xylose nor a xylose metabolic pathway has been described. The aims of this study were to identify and characterize the xylose metabolism pathway of T18 and, through genetic engineering, develop a strain capable of growth on hemicellulosic sugars.
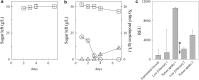
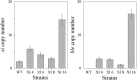
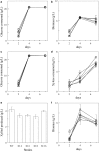
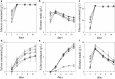
Results: Characterization of T18 performance in glucose/xylose media revealed diauxic growth and copious extracellular xylitol production. Furthermore, T18 did not grow in media containing xylose as the only carbon source. We identified, cloned, and functionally characterized a xylose isomerase. Transcriptomics indicated that this xylose isomerase gene is upregulated when xylose is consumed by the cells. Over-expression of the native xylose isomerase in T18, creating strain XI 16, increased xylose consumption from 5.2 to 7.6 g/L and reduced extracellular xylitol from almost 100% to 68%. Xylose utilization efficiency of this strain was further enhanced by over-expressing a heterologous xylulose kinase to reduce extracellular xylitol to 20%. Moreover, the ability to grow in media containing xylose as a sole sugar was dependent on the copy number of both xylose isomerase and xylulose kinase present. In fed-batch fermentations, the best xylose metabolizing isolate, XI-XK 7, used 137 g of xylose versus 39 g by wild type and produced more biomass and fatty acid.
Conclusions: The presence of a typically prokaryotic xylose isomerase and xylitol production through a typically eukaryotic xylose reductase pathway in T18 is the first report of an organism naturally encoding enzymes from two native xylose metabolic pathways. Our newly engineered strains pave the way for the growth of T18 on waste hemicellulosic feedstocks for biofuel production.
Keywords: Biomass production; Lipid production for biofuel; Metabolic engineering; Thraustochytrid; Xylose isomerase; Xylose metabolism.
Figures




References
-
- Scaife MA, Merkx-Jacques A, Woodhall DL, Armenta RE. Algal biofuels in Canada: status and potential. Renew Sustain Energy Rev. 2015;44:620–642.
-
- Sarkar D, Shimizu K. An overview on biofuel and biochemical production by photosynthetic microorganisms with understanding of the metabolism and by metabolic engineering together with efficient cultivation and downstream processing. Bioresour Bioprocess. 2015;2:17.
-
- González-Rosas A, Miranda-Gómez JM, Padmasree KP, Fernández-Luqueño F. How green is bioenergy? A review on myths, challenges, biotechnology progress and emerging possibilities. Sci Res Essays. 2013;8:532–542.
-
- Armenta RE, Sun Z. Heterotrophic culturing of microalgae. Microalgal production biomass high-value products. Boca Raton: CRC Press; 2016. pp. 311–325.
LinkOut - more resources
Full Text Sources
Other Literature Sources

