Biochemical and cellular consequences of the antithrombin p.Met1? mutation identified in a severe thrombophilic family
- PMID: 30237862
- PMCID: PMC6145704
- DOI: 10.18632/oncotarget.26059
Biochemical and cellular consequences of the antithrombin p.Met1? mutation identified in a severe thrombophilic family
Abstract
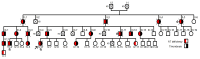
Nature is always the best inspiration for basic research. A family with severe thrombosis and antithrombin deficiency, the strongest anticoagulant, carried a new mutation affecting the translation-start codon of SERPINC1, the gene encoding antithrombin. Expression of this variant in a eukaryotic cell system produced three different antithrombins. Two downstream methionines were used as alternative initiation codons, generating highly expressed small aglycosylated antithrombins with cytoplasmic localization. Wild-type antithrombin was generated by the use of the mutated AUU as initiation codon. Actually, any codon except for the three stop codons might be used to initiate translation in this strong Kozak context. We show unexpected consequences of natural mutations affecting translation-start codons. Downstream alternative initiation AUG codons may be used when the start codon is mutated, generating smaller molecules with potential different cell localization, biochemical features and unexplored consequences. Additionally, our data further support the use of other codons apart from AUG for initiation of translation in eukaryotes.
Keywords: antithrombin; initiation codon; thrombosis; translation.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures





References
-
- Lane DA, Bayston T, Olds RJ, Fitches AC, Cooper DN, Millar DS, Jochmans K, Perry DJ, Okajima K, Thein SL, Emmerich J, Plasma Coagulation Inhibitors Subcommittee of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis Antithrombin mutation database: 2nd (1997) update. Thromb Haemost. 1997;77:197–211. doi: 10.1055/s-0038-1655930. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

