Race and Hepatitis C Care Continuum in an Underserved Birth Cohort
- PMID: 30238404
- PMCID: PMC6816604
- DOI: 10.1007/s11606-018-4649-6
Race and Hepatitis C Care Continuum in an Underserved Birth Cohort
Abstract
Background: Birth cohort screening is recommended for hepatitis C virus (HCV) and underserved populations are disproportionally affected by HCV. Little is known about the influence of race on the HCV care continuum in this population.
Objective: To assess the cascade of HCV care in a large racially diverse and underserved birth cohort.
Design: Retrospective cohort study using electronic medical record data abstracted until August 31, 2017.
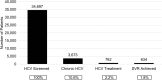
Patients: 34,810 patients born between 1945 and 1965 engaged in primary care between October 1, 2014, and October 31, 2016, within the safety-net clinics of the San Francisco Health Network.
Main measures: Rate of hepatitis C testing, hepatitis C treatment, and response to therapy.
Results: Cohort characteristics were as follows: median age 59 years, 57.6% male, 25.5% White (20.6% Black, 17.7% Latino, 33.0% Asian/Pacific Islander (API), 2% other), and 32.6% preferred a non-English language. 99.7% had an HCV test (95.4% HCV antibody, 4.3% HCVRNA alone). Among HCV antibody-positive patients (N = 4587), 22.9% were not tested for confirmatory HCVRNA. Among viremic patients (N = 3673), 20.8% initiated HCV therapy, 90.6% achieved sustained virologic response (SVR) and 8.1% did not have a SVR test. HCV screening and treatment were highest in APIs (98.7 and 34.7% respectively; p < 0.001). Blacks had the highest chronic HCV rate (22.2%; p < 0.001). Latinos had the lowest SVR rate (81.3%; p = 0.01). On multivariable analysis, API race (vs White, OR 1.20; p = 0.001), presence of HIV co-infection (OR 1.58; p = 0.02), presence of chronic kidney disease (OR 0.47; p < 0.001), English (vs non-English) as preferred language (OR 0.54; p = 0.002), ALT (OR 0.39 per doubling; p < 0.001), and HCVRNA (OR 0.83 per 10-fold increase; p < 0.001) were associated with HCV treatment.
Conclusions: Despite near-universal screening, gaps in active HCV confirmation, treatment, and verification of cure were identified and influenced by race. Tailored interventions to engage and treat diverse and underserved populations with HCV infection are needed.
Keywords: African American; direct-acting antivirals; health disparity; linkage to care; vulnerable populations.
Conflict of interest statement
The authors do not have any conflicts of interest to report in connection with this manuscript.
Dr. Khalili’s full disclosure includes research grant funding (to her institution) from Gilead Sciences Inc., Intercept Pharmaceuticals, and Abbvie, and she has served on the scientific advisory boards of Gilead Sciences Inc., and Intercept Pharmaceuticals.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

