Biocompatibility of Polysulfone Hemodialysis Membranes and Its Mechanisms: Involvement of Fibrinogen and Its Integrin Receptors in Activation of Platelets and Neutrophils
- PMID: 30239013
- PMCID: PMC6220809
- DOI: 10.1111/aor.13268
Biocompatibility of Polysulfone Hemodialysis Membranes and Its Mechanisms: Involvement of Fibrinogen and Its Integrin Receptors in Activation of Platelets and Neutrophils
Abstract
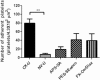
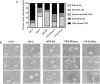
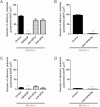
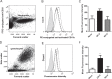
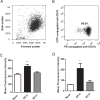
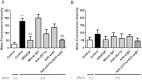
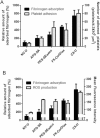
Activation of blood cells during hemodialysis is considered to be a significant determinant of biocompatibility of the hemodialysis membrane because it may affect patient health adversely through microvascular inflammation and oxidative stress. This study found very different cell activation among various polysulfone (PSf) hemodialysis membranes. For example, CX-U, a conventional PSf membrane, induced marked adhesion of platelets to its surface and increased surface expression of activated CD11b and production of reactive oxygen species (ROS) by neutrophils; while NV-U, a hydrophilic polymer-immobilized PSf membrane, caused little platelet adhesion and slight CD11b expression and ROS production by neutrophils. Analysis of the molecular mechanisms of the above phenomena on CX-U and NV-U indicated that anti-integrin GPIIb/IIIa antibody blocked platelet adhesion, and that the combination of anti-CD11b (integrin α subunit of Mac-1) and anti-integrin αvβ3 antibodies blocked ROS production by neutrophils. Plasma-derived fibrinogen, a major ligand of GPIIb/IIIa, Mac-1, and αvβ3 on membranes, was thus analyzed and found to be more adsorbed to CX-U than to NV-U. Moreover, comparison between five PSf membranes showed that the number of adherent platelets and neutrophil ROS production increased with increasing fibrinogen adsorption. These results suggested that fibrinogen, adsorbed on membranes, induced GPIIb/IIIa-mediated platelet activation and Mac-1/αvβ3-mediated neutrophil activation, depending on the amount of adsorption. In conclusion, the use of biocompatible membranes like NV-U, which show lower adsorption of fibrinogen, is expected to reduce hemodialysis-induced inflammation and oxidative stress by minimizing cell activation.
Keywords: Biocompatibility; Fibrinogen; Hemodialysis membrane; Neutrophil; Platelet.
© 2018 The Authors. Artificial Organs published by Wiley Periodicals, Inc. on behalf of International Center for Artificial Organ and Transplantation (ICAOT).
Figures

References
-
- Daugirdas JT, Bernardo AA. Hemodialysis effect on platelet count and function and hemodialysis‐associated thrombocytopenia. Kidney Int 2012;82:147–57. - PubMed
-
- Sirolli V, Ballone E, Di Stante S, Amoroso L, Bonomini M. Cell activation and cellular‐cellular interactions during hemodialysis: effect of dialyzer membrane. Int J Artif Organs 2002;25:529–37. - PubMed
-
- Yoon JW, Pahl MV, Vaziri ND. Spontaneous leukocyte activation and oxygen‐free radical generation in end‐stage renal disease. Kidney Int 2007;71:167–72. - PubMed
-
- Pertosa G, Grandaliano G, Gesualdo L, Schena FP. Clinical relevance of cytokine production in hemodialysis. Kidney Int Suppl 2000;58:S104–11. - PubMed
-
- Kakuta T, Komaba H, Takagi N, et al. A prospective multicenter randomized controlled study on interleukin‐6 removal and induction by a new hemodialyzer with improved biocompatibility in HD patients: a pilot study. Ther Apher Dial 2016;20:569–78. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

