Emerging structural biology of lipid G protein-coupled receptors
- PMID: 30239054
- PMCID: PMC6319753
- DOI: 10.1002/pro.3509
Emerging structural biology of lipid G protein-coupled receptors
Abstract
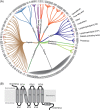
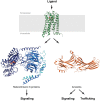
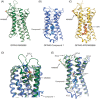
The first crystal structure of a G protein-coupled receptor (GPCR) was that of the bovine rhodopsin, solved in 2000, and is a light receptor within retina rode cells that enables vision by transducing a conformational signal from the light-induced isomerization of retinal covalently bound to the receptor. More than 7 years after this initial discovery and following more than 20 years of technological developments in GPCR expression, stabilization, and crystallography, the high-resolution structure of the adrenaline binding β2 -adrenergic receptor, a ligand diffusible receptor, was discovered. Since then, high-resolution structures of more than 53 unique GPCRs have been determined leading to a significant improvement in our understanding of the basic mechanisms of ligand-binding and ligand-mediated receptor activation that revolutionized the field of structural molecular pharmacology of GPCRs. Recently, several structures of eight unique lipid-binding receptors, one of the most difficult GPCR families to study, have been reported. This review presents the outstanding structural and pharmacological features that have emerged from these new lipid receptor structures. The impact of these findings goes beyond mechanistic insights, providing evidence of the fundamental role of GPCRs in the physiological integration of the lipid signaling system, and highlighting the importance of sustained research into the structural biology of GPCRs for the development of new therapeutics targeting lipid receptors.
Keywords: G protein-coupled receptor; allosteric ligands; bioactive lipids; crystal structures; membrane proteins.
© 2018 The Protein Society.
Figures




References
-
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M (2000) Crystal structure of rhodopsin: A G protein‐coupled receptor. Science 289:739–745. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

