White matter diffusion alterations precede symptom onset in autosomal dominant Alzheimer's disease
- PMID: 30239611
- PMCID: PMC6158739
- DOI: 10.1093/brain/awy229
White matter diffusion alterations precede symptom onset in autosomal dominant Alzheimer's disease
Abstract
White matter alterations are present in the majority of patients with Alzheimer's disease type dementia. However, the spatiotemporal pattern of white matter changes preceding dementia symptoms in Alzheimer's disease remains unclear, largely due to the inherent diagnostic uncertainty in the preclinical phase and increased risk of confounding age-related vascular disease and stroke in late-onset Alzheimer's disease. In early-onset autosomal-dominantly inherited Alzheimer's disease, participants are destined to develop dementia, which provides the opportunity to assess brain changes years before the onset of symptoms, and in the absence of ageing-related vascular disease. Here, we assessed mean diffusivity alterations in the white matter in 64 mutation carriers compared to 45 non-carrier family non-carriers. Using tract-based spatial statistics, we mapped the interaction of mutation status by estimated years from symptom onset on mean diffusivity. For major atlas-derived fibre tracts, we determined the earliest time point at which abnormal mean diffusivity changes in the mutation carriers were detectable. Lastly, we assessed the association between mean diffusivity and cerebrospinal fluid biomarkers of amyloid, tau, phosphorylated-tau, and soluble TREM2, i.e. a marker of microglia activity. Results showed a significant interaction of mutations status by estimated years from symptom onset, i.e. a stronger increase of mean diffusivity, within the posterior parietal and medial frontal white matter in mutation carriers compared with non-carriers. The earliest increase of mean diffusivity was observed in the forceps major, forceps minor and long projecting fibres-many connecting default mode network regions-between 5 to 10 years before estimated symptom onset. Higher mean diffusivity in fibre tracts was associated with lower grey matter volume in the tracts' projection zones. Global mean diffusivity was correlated with lower cerebrospinal fluid levels of amyloid-β1-42 but higher levels of tau, phosphorylated-tau and soluble TREM2. Together, these results suggest that regionally selective white matter degeneration occurs years before the estimated symptom onset. Such white matter alterations are associated with primary Alzheimer's disease pathology and microglia activity in the brain.
Figures




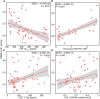
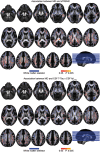
Comment in
-
Where do white matter alterations dovetail with the cascade model of Alzheimer's disease?Brain. 2018 Oct 1;141(10):2830-2833. doi: 10.1093/brain/awy243. Brain. 2018. PMID: 30535117 No abstract available.
References
-
- Acosta-Cabronero J, Williams GB, Pengas G, Nestor PJ. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer’s disease. Brain 2010; 133 (Pt 2): 529–39. - PubMed
-
- Agosta F, Pievani M, Sala S, Geroldi C, Galluzzi S, Frisoni GB et al. White matter damage in Alzheimer disease and its relationship to gray matter atrophy. Radiology 2011; 258: 853–63. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

