Effects of Pitavastatin on Insulin Sensitivity and Liver Fat: A Randomized Clinical Trial
- PMID: 30239757
- PMCID: PMC6194811
- DOI: 10.1210/jc.2018-01446
Effects of Pitavastatin on Insulin Sensitivity and Liver Fat: A Randomized Clinical Trial
Abstract
Context: 3-Hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitors (statins) are widely prescribed. Statins may have important metabolic effects on insulin sensitivity and liver fat, but limited studies have assessed these effects by using euglycemic hyperinsulinemic clamp, stable isotopes, and 1H magnetic resonance spectroscopy (MRS) for liver fat quantification.
Objective: To study the effects of pitavastatin on hepatic fat and insulin sensitivity.
Design: Six-month, double-blind, randomized, placebo-controlled trial.
Setting: Academic clinical research center in Boston, Massachusetts.
Participants: Overweight, insulin-resistant men aged 40 to 65 years who had not received statin therapy for ≥1 year.
Interventions: Pitavastatin 4 mg or placebo daily.
Outcome: The primary endpoints were changes in insulin sensitivity measured by euglycemic hyperinsulinemic clamp and liver fat measured by 1H MRS.
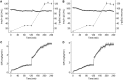
Results: Pitavastatin showed no effect on endogenous glucose production (ΔRa glucose 0.07 ± 0.07 vs 0.04 ± 0.07 mg/kg/min, pitavastatin vs placebo, P = 0.76) or insulin-stimulated glucose uptake during "low dose" (ΔM 0.1 ± 0.1 vs -0.3 ± 0.2 mg/kg/min, P = 0.11) and "high dose" (ΔM -0.5 ± 0.3 vs -0.7 ± 0.4 mg/kg/min, P = 0.70) euglycemic hyperinsulinemic clamps. There was also no effect of pitavastatin on fasting glucose, HbA1c, and 2-hour glucose after 75-g glucose challenge. There was also no change in liver fat fraction (-1 ± 1 vs -0 ± 1%, P = 0.56).
Conclusion: Compared with placebo, pitavastatin did not affect hepatic or whole-body insulin sensitivity, and it did not reduce liver fat.
Trial registration: ClinicalTrials.gov NCT02290106.
Figures

References
-
- Bhandari S, Gupta P, Quinn P, Sandhu J, Hakimi A, Jones D, Ng L. Pleiotropic effects of statins in hypercholesterolaemia: a prospective observational study using a lipoproteomic based approach. Lancet. 2015;385(suppl 1):S21. - PubMed
-
- Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH; CARDS investigators . Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–696. - PubMed
-
- Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J; ASCOT Investigators . Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Drugs. 2004;64(suppl 2):43–60. - PubMed
-
- Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375(9716):735–742. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

