Structural insights into the mechanism of double strand break formation by Hermes, a hAT family eukaryotic DNA transposase
- PMID: 30239795
- PMCID: PMC6212770
- DOI: 10.1093/nar/gky838
Structural insights into the mechanism of double strand break formation by Hermes, a hAT family eukaryotic DNA transposase
Abstract
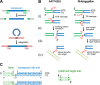
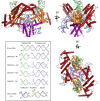
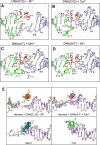
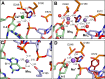
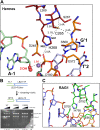
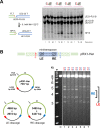
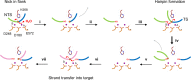
Some DNA transposons relocate from one genomic location to another using a mechanism that involves generating double-strand breaks at their transposon ends by forming hairpins on flanking DNA. The same double-strand break mode is employed by the V(D)J recombinase at signal-end/coding-end junctions during the generation of antibody diversity. How flanking hairpins are formed during DNA transposition has remained elusive. Here, we describe several co-crystal structures of the Hermes transposase bound to DNA that mimics the reaction step immediately prior to hairpin formation. Our results reveal a large DNA conformational change between the initial cleavage step and subsequent hairpin formation that changes which strand is acted upon by a single active site. We observed that two factors affect the conformational change: the complement of divalent metal ions bound by the catalytically essential DDE residues, and the identity of the -2 flanking base pair. Our data also provides a mechanistic link between the efficiency of hairpin formation (an A:T basepair is favored at the -2 position) and Hermes' strong target site preference. Furthermore, we have established that the histidine residue within a conserved C/DxxH motif present in many transposase families interacts directly with the scissile phosphate, suggesting a crucial role in catalysis.
Figures
References
-
- Gillings M.R., Paulsen I.T., Tetu S.G.. Genomics and the evolution of antibiotic resistance. Ann. N. Y. Acad. Sci. 2017; 1388:92–107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

