An autopsy report of three kindred in a Gerstmann-Sträussler-Scheinker disease P105L family with a special reference to prion protein, tau, and beta-amyloid
- PMID: 30240140
- PMCID: PMC6192393
- DOI: 10.1002/brb3.1117
An autopsy report of three kindred in a Gerstmann-Sträussler-Scheinker disease P105L family with a special reference to prion protein, tau, and beta-amyloid
Abstract
Introduction: Gerstmann-Sträussler-Scheinker disease P105L (GSS105) is a rare variant of GSS caused by a point mutation of the prion protein (PrP) gene at codon 105 (proline to leucine substitution). It is clinically characterized by spastic paraparesis and dementia and histopathologically defined by PrP-plaques in the brain. This report describes a clinicopathological analysis of three autopsied kindred from a Japanese GSS105 family, plus a topological analysis of PrP, hyperphosphorylated tau (p-tau), and beta-amyloid (Aβ).
Methods: Using paraffin-embedded sections, we applied histology and single- and multiple-labeling immunohistochemistry for PrP, p-tau, and Aβ to the three cases. Comparative semi-quantitative analyses of tissue injuries and PrP-plaques were also employed.
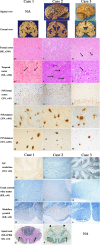
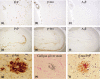
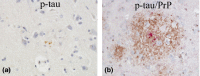
Results: Case 1 (45 years old (yo)) and Case 2 (56 yo) are sisters, and Case 3 (49 yo) is the son of Case 2. Case 1 and Case 2 presented with spastic paraparesis followed by dementia, whereas Case 3 presented, not with spastic paraparesis, but with psychiatric symptoms. In Case 1 and Case 2, the brain showed tissue injuries with many PrP-plaques in the cerebral cortices, and the pyramidal tract showed myelin loss/pallor. In Case 3, the brain was least degenerated with a number of PrP-plaques; however, the pyramidal tract remained intact. In addition, p-tau was deposited in all cases, where p-tau was present in or around PrP-plaques. By double-labeling immunohistochemistry, the colocalization of p-tau with PrP-plaques was confirmed. Moreover in Case 2, Aβ was deposited in the cerebral cortices. Interestingly, not only p-tau but also Aβ was colocalized with PrP-plaques. In all cases, both three repeat tau and four repeat tau were associated with PrP-plaques.
Conclusions: The clinicopathological diversity of GSS105, which is possible even in the same family, was ascertained. Not only p-tau but also Aβ could be induced by PrP ("secondary degeneration"), facilitating the kaleidoscopic symptoms of GSS.
Keywords: Gerstmann-Sträussler-Scheinker disease P105L; autopsy; beta-amyloid; prion protein; spastic paraparesis; tau.
© 2018 The Authors. Brain and Behavior published by Wiley Periodicals, Inc.
Figures



References
-
- Alzualde, A. , Indakoetxea, B. , Ferrer, I. , Moreno, F. , Barandiaran, M. , Gorostidi, A. , … Lopez de Munain, A. (2010). A novel PRNP Y218N mutation in Gerstmann‐Sträussler‐Scheinker disease with neurofibrillary degeneration. Journal of Neuropathology & Experimental Neurololgy, 69, 789–800. 10.1097/NEN.0b013e3181e85737. - DOI - PubMed
-
- Amano, N. , Yagishita, S. , Yokoi, S. , Itoh, Y. , Kinoshita, J. , Mizutani, T. , … Matsuishi, T. (1992). Gerstmann‐Sträussler syndrome‐A variant type: Amyloid plaques and Alzheimer's neurofibrillary tangles in cerebral cortex. Acta Neuropathologica, 84, 15–23. - PubMed
-
- Bugiani, O. , Giaccone, G. , Verga, L. , Pollo, B. , Frangione, B. , Farlow, M. R. , … Ghetti, B. (1993). Beta PP participates in PrP‐amyloid plaques of Gerstmann‐Sträussler‐Scheinker disease, Indiana kindred. Journal of Neuropathology & Experimental Neurology, 52, 64–70. - PubMed
-
- Colucci, M. , Moleres, F. J. , Xie, Z. L. , Ray‐Chaudhury, A. , Gutti, S. , Butefisch, C. M. , … Gambetti, P. (2006). Gerstmann‐Sträussler‐Scheinker: A new phenotype with 'curly' PrP deposits. Journal of Neuropathology & Experimental Neurology, 65, 642–651. 10.1097/01.jnen.0000228198.81797.4d - DOI - PubMed
-
- de Silva, R. , Lashley, T. , Gibb, G. , Hanger, D. , Hope, A. , Reid, A. , … Lees, A. (2003). Pathological inclusion bodies in tauopathies contain distinct complements of tau with three or four microtubule‐binding repeat domains as demonstrated by new specific monoclonal antibodies. Neuropathology and Applied Neurobiology, 29, 288–302. 10.1046/j.1365-2990.2003.00463.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

