Longitudinal Trajectory and Characterization of Cancer-Related Cognitive Impairment in a Nationwide Cohort Study
- PMID: 30240328
- PMCID: PMC6225503
- DOI: 10.1200/JCO.2018.78.6624
Longitudinal Trajectory and Characterization of Cancer-Related Cognitive Impairment in a Nationwide Cohort Study
Abstract
Purpose: Cancer-related cognitive impairment (CRCI) is an important clinical problem in patients with breast cancer receiving chemotherapy. Nationwide longitudinal studies are needed to understand the trajectory and severity of CRCI in specific cognitive domains.
Patients and methods: The overall objective of this nationwide, prospective, observational study conducted within the National Cancer Institute Community Clinical Oncology Research Program was to assess trajectories in specific cognitive domains in patients with breast cancer (stage I-IIIC) receiving chemotherapy, from pre- (A1) to postchemotherapy (A2) and from prechemotherapy to 6 months postchemotherapy (A3); controls were assessed at the same time-equivalent points. The primary aim assessed visual memory using the Cambridge Neuropsychological Test Automated Battery Delayed Match to Sample test by longitudinal mixed models including A1, A2, and A3 and adjusting for age, education, race, cognitive reserve score, and baseline anxiety and depressive symptoms. We also assessed trajectories of CRCI in other aspects of memory as well as in attention and executive function with computerized, paper-based, and telephone-based cognitive tests.
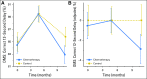
Results: In total, 580 patients with breast cancer (mean age, 53.4 years) and 363 controls (mean age, 52.6 years) were assessed. On the Delayed Match to Sample test, the longitudinal mixed model results revealed a significant group-by-time effect ( P < .005); patients declined over time from prechemotherapy (A1) to 6 months postchemotherapy (A3; P = .005), but controls did not change ( P = .426). The group difference between patients and controls was also significant, revealing declines in patients but not controls ( P = .017). Several other models of computerized, standard, and telephone tests indicated significantly worse performance by patients compared with controls from pre- to postchemotherapy and from prechemotherapy to 6 months postchemotherapy.
Conclusion: This nationwide study showed CRCI in patients with breast cancer affects multiple cognitive domains for at least 6 months postchemotherapy.
Figures


References
-
- Brezden CB, Phillips KA, Abdolell M, et al. : Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol 18:2695-2701, 2000 - PubMed
-
- Ahles TA, Saykin AJ, Furstenberg CT, et al. : Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol 20:485-493, 2002 - PubMed
-
- van Dam FS, Schagen SB, Muller MJ, et al. : Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: High-dose versus standard-dose chemotherapy. J Natl Cancer Inst 90:210-218, 1998 - PubMed
-
- Wefel JS, Lenzi R, Theriault RL, et al. : The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: Results of a prospective, randomized, longitudinal trial. Cancer 100:2292-2299, 2004 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

