Three-dimensional culture of chicken primordial germ cells (cPGCs) in defined media containing the functional polymer FP003
- PMID: 30240390
- PMCID: PMC6150485
- DOI: 10.1371/journal.pone.0200515
Three-dimensional culture of chicken primordial germ cells (cPGCs) in defined media containing the functional polymer FP003
Abstract
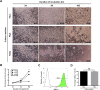
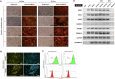
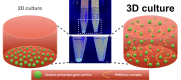
Scalable production of avian cell lines exhibits a valuable potential on therapeutic application by producing recombinant proteins and as the substrate for virus growth due to the special glycosylation occurs in avian species. Chicken primordial germ cells (cPGCs), a germinal pluripotent avian cell type, present the ability of self-renewal, an anchorage-independent cell growth and the ability to be genetically modified. This cell type could be an interesting bioreactor system for industrial purposes. This study sought to establish an expandable culture system with defined components for three-dimensional (3D) culture of cPGCs. cPGCs were cultured in medium supplemented with the functional polymer FP003. Viscoelasticity was low in this medium but cPGCs did not sediment in culture and efficiencies of space and nutrient utilization were thus enhanced and consequently their expansion was improved. The total number of cPGCs increased by 17-fold after 1 week of culture in 3D-FAot medium, an aseric defined medium containing FP003 polymer, FGF2 and Activin A as growth factors and Ovotransferrin as protein. Moreover, cPGC cell lines stably expressed the germline-specific reporter VASA:tdTOMATO, as well as other markers of cPGCs, for more than 1 month upon culture in 3D-FAot medium, indicating that the characteristics of these cells are maintained. In summary, this novel 3D culture system can be used to efficiently expand cPGCs in suspension without mechanical stirring, which is available for long-term culture and no loss of cellular properties was found. This system provides a platform for large-scale culture of cPGCs.
Conflict of interest statement
Nissan Chemical Corporation provided support in the form of salaries for authors MM, HH, and TK, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures




References
-
- Hiroki O, Eri N, Anna N, Hiroshi Y, Tadahiro H, Takahiro I, et al. Effective Transplantation of 2D and 3D Cultured Hepatocyte Spheroids Confirmed by Quantum Dot Imaging. Advanced Biosystems. 2018;0(0):1800137.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

