Applications of mechanistic modelling to clinical and experimental immunology: an emerging technology to accelerate immunotherapeutic discovery and development
- PMID: 30240512
- PMCID: PMC6150250
- DOI: 10.1111/cei.13182
Applications of mechanistic modelling to clinical and experimental immunology: an emerging technology to accelerate immunotherapeutic discovery and development
Abstract
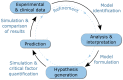
The application of in-silico modelling is beginning to emerge as a key methodology to advance our understanding of mechanisms of disease pathophysiology and related drug action, and in the design of experimental medicine and clinical studies. From this perspective, we will present a non-technical discussion of a small number of recent and historical applications of mathematical, statistical and computational modelling to clinical and experimental immunology. We focus specifically upon mechanistic questions relating to human viral infection, tumour growth and metastasis and T cell activation. These exemplar applications highlight the potential of this approach to impact upon human immunology informed by ever-expanding experimental, clinical and 'omics' data. Despite the capacity of mechanistic modelling to accelerate therapeutic discovery and development and to de-risk clinical trial design, it is not utilized widely across the field. We outline ongoing challenges facing the integration of mechanistic modelling with experimental and clinical immunology, and suggest how these may be overcome. Advances in key technologies, including multi-scale modelling, machine learning and the wealth of 'omics' data sets, coupled with advancements in computational capacity, are providing the basis for mechanistic modelling to impact on immunotherapeutic discovery and development during the next decade.
© 2018 British Society for Immunology.
Figures

References
-
- Claret L, Girard P, Hoff P M et al Model‐based prediction of phase III overall survival in colorectal cancer on the basis of phase II tumor dynamics. J Clin Oncol 2009; 27: 4103–8. - PubMed
-
- Food and Drug Administration (FDA) . Innovation or Stagnation: Challenge and Opportunity on the Critical Path to New Medical Products. Silver Spring, MD: FDA, 2004.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

