Towards high-throughput fast photochemical oxidation of proteins: Quantifying exposure in high fluence microtiter plate photolysis
- PMID: 30240591
- PMCID: PMC6186496
- DOI: 10.1016/j.ab.2018.09.014
Towards high-throughput fast photochemical oxidation of proteins: Quantifying exposure in high fluence microtiter plate photolysis
Abstract
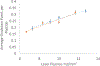
Protein structural analysis by mass spectrometry has gained significant popularity in recent years, including high-resolution protein topographical mapping by fast photochemical oxidation of proteins (FPOP). The ability to provide protein topographical information at moderate spatial resolution makes FPOP an attractive technology for the protein pharmaceutical discovery and development processes. However, current technology limits the throughput and requires significant manual sample manipulation. Similarly, as FPOP is being used on larger samples, sample flow through the capillary becomes challenging. No systematic comparison of the performance of static flash photolysis with traditional flow FPOP has been reported. Here, we evaluate a 96-well microtiter-based laser flash photolysis method for the topographical probing of proteins, which subsequently could be used to analyze higher order structure of the protein in a high-throughput fashion with minimal manual sample manipulation. We used multiple metrics to compare microtiter FPOP performance with that of traditional flow FPOP: adenine-based hydroxyl radical dosimetry, oxidation efficiency of a model peptide, and hydroxyl radical protein footprint of myoglobin. In all cases, microtiter plate FPOP performed comparably with traditional flow FPOP, requiring a small fraction of the time for exposure. This greatly reduced sample exposure time, coupled with automated sample handling in 96-well microtiter plates, makes microtiter-based FPOP an important step in achieving the throughput required to adapt hydroxyl radical protein footprinting for screening purposes.
Keywords: Covalent labeling; Fast photochemical oxidation of proteins (FPOP); Hydroxyl radical protein footprinting (HRPF); Mass spectrometry; Myoglobin.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Disclosure
J.S.S. discloses a significant financial interest in GenNext Technologies, Inc., a small company seeking to commercialize technologies for protein higher order structure analysis.
Figures
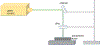
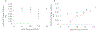

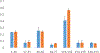
References
-
- Konermann L, Vahidi S, Sowole MA, Mass spectrometry methods for studying structure and dynamics of biological macromolecules, Anal. Chem, 86 (2013) 213–232. - PubMed
-
- Sharp JS, Becker JM, Hettich RL, Protein surface mapping by chemical oxidation: Structural analysis by mass spectrometry, Anal. Biochem, 313 (2003) 216–225. - PubMed
-
- Hambly DM, Gross ML, Laser flash photolysis of hydrogen peroxide to oxidize protein solvent-accessible residues on the microsecond timescale, J. Am. Soc. Mass Spectrom, 16 (2005) 2057–2063. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

