Practical Enantioselective Reduction of Ketones Using Oxazaborolidine Catalysts Generated In Situ from Chiral Lactam Alcohols
- PMID: 30241305
- PMCID: PMC6222374
- DOI: 10.3390/molecules23102408
Practical Enantioselective Reduction of Ketones Using Oxazaborolidine Catalysts Generated In Situ from Chiral Lactam Alcohols
Abstract
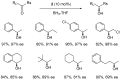
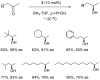
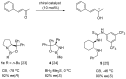
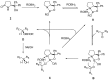
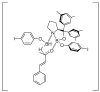
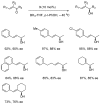
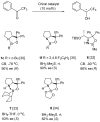
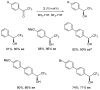
Oxazaborolidine catalyst (CBS catalyst) has been extensively used for catalytic borane reduction with a predictable absolute stereochemistry and high enantioselectivity. However, the use of isolated CBS catalyst sometimes has the drawback of low reproducibility due to the aging of the CBS catalyst during storage. Therefore, we investigated a more reliable and practical method for the reduction of a variety of ketones including challenging substrates, primary aliphatic ketones, α,β-enones, and trifluoromethyl ketones. This review surveys the developments in borane reduction using oxazaborolidine catalysts generated in situ from chiral lactam alcohols and borane.
Keywords: asymmetric synthesis; borane; enantioselective; lactam alcohol; reduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
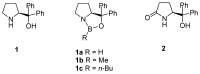

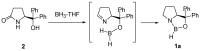
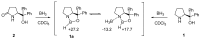
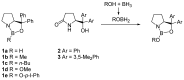



References
-
- Wallbaum S., Martens J. Asymmetric syntheses with chiral oxazaborolidines. Tetrahedron Asymmetry. 1992;3:1475–1504. doi: 10.1016/S0957-4166(00)86044-9. - DOI
-
- Singh V.K. Practical and useful methods for the enantioselective reduction of unsymmetrical ketones. Synthesis. 1992:605–620. doi: 10.1055/s-1992-26174. - DOI
-
- Deloux L., Srebnik M. Asymmetric boron-catalyzed reactions. Chem. Rev. 1993;93:763–777. doi: 10.1021/cr00018a007. - DOI
-
- Corey E.J., Helal C.J. Reduction of carbonyl compounds with chiral oxazaborolidine catalysts: A new paradigm for enantioselective catalysis and a powerful new synthetic method. Angew. Chem. Int. Ed. 1998;37:1986–2012. doi: 10.1002/(SICI)1521-3773(19980817)37:15<1986::AID-ANIE1986>3.0.CO;2-Z. - DOI - PubMed
-
- Hirao A., Itsuno S., Nakahama S., Yamazaki N. Asymmetric reduction of aromatic ketones with chiral alkoxy-amine-borane complexes. J. Chem. Soc. Chem. Commun. 1981;0:315–317. doi: 10.1039/c39810000315. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

