RNA-seq analysis of gene expression changes during pupariation in Bactrocera dorsalis (Hendel) (Diptera: Tephritidae)
- PMID: 30241467
- PMCID: PMC6150976
- DOI: 10.1186/s12864-018-5077-z
RNA-seq analysis of gene expression changes during pupariation in Bactrocera dorsalis (Hendel) (Diptera: Tephritidae)
Abstract
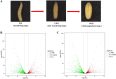
Background: The oriental fruit fly, Bactrocera dorsalis (Hendel) has been considered to be one of the most important agricultural pest around the world. As a holometabolous insect, larvae must go through a metamorphosis process with dramatic morphological and structural changes to complete their development. To better understand the molecular mechanisms of these changes, RNA-seq of B. dorsalis from wandering stage (WS), late wandering stage (LWS) and white puparium stage (WPS) were performed.
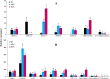
Results: In total, 11,721 transcripts were obtained, out of which 1914 genes (578 up-regulated and 1336 down-regulated) and 2047 genes (655 up-regulated and 1392 down-regulated) were found to be differentially expressed between WS and LWS, as well as between WS and WPS, respectively. Of these DEGs, 1862 and 1996 genes were successfully annotated in various databases. The analysis of RNA-seq data together with qRT-PCR validation indicated that during this transition, the genes in the oxidative phosphorylation pathway, and genes encoding P450s, serine protease inhibitor, and cuticular proteins were down-regulated, while the serine protease genes were up-regulated. Moreover, we found some 20-hydroxyecdysone (20E) biosynthesis and signaling pathway genes had a higher expression in the WS, while the genes responsible for juvenile hormone (JH) synthesis, degradation, signaling and transporter pathways were down-regulated, suggesting these genes might be involved in the process of larval pupariation in B. dorsalis. For the chitinolytic enzymes, the genes encoding chitinases (chitinase 2, chitinase 5, chitinase 8, and chitinase 10) and chitin deacetylase might play the crucial role in the degradation of insect chitin with their expressions significantly increased during the transition. Here, we also found that chitin synthase 1A might be involved in the chitin synthesis of cuticles during the metamorphosis in B. dorsalis.
Conclusions: Significant changes at transcriptional level were identified during the larval pupariation of B. dorsalis. Importantly, we also obtained a vast quantity of RNA-seq data and identified metamorphosis associated genes, which would all help us to better understand the molecular mechanism of metamorphosis process in B. dorsalis.
Keywords: Bactrocera dorsalis; Gene expression; Metamorphosis; Pupariation; RNA-Seq.
Conflict of interest statement
Ethics approval and consent to participate
No special permits were required to collect oriental fruit flies.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




Similar articles
-
Two Chitin Biosynthesis Pathway Genes in Bactrocera dorsalis (Diptera: Tephritidae): Molecular Characteristics, Expression Patterns, and Roles in Larval-Pupal Transition.J Econ Entomol. 2015 Oct;108(5):2433-42. doi: 10.1093/jee/tov186. Epub 2015 Jul 1. J Econ Entomol. 2015. PMID: 26453732
-
RNA-seq analysis of gene expression changes in cuticles during the larval-pupal metamorphosis of Plutella xylostella.Comp Biochem Physiol Part D Genomics Proteomics. 2021 Sep;39:100869. doi: 10.1016/j.cbd.2021.100869. Epub 2021 Jun 19. Comp Biochem Physiol Part D Genomics Proteomics. 2021. PMID: 34171685
-
Potential targets for controlling Bactrocera dorsalis using cuticle- and hormone-related genes revealed by a developmental transcriptome analysis.Pest Manag Sci. 2020 Jun;76(6):2127-2143. doi: 10.1002/ps.5751. Epub 2020 Feb 14. Pest Manag Sci. 2020. PMID: 31951094
-
Insect metamorphosis and chitin metabolism under miRNA regulation: a review with current advances.Pest Manag Sci. 2025 Jul;81(7):3437-3451. doi: 10.1002/ps.8758. Epub 2025 Mar 13. Pest Manag Sci. 2025. PMID: 40079237 Review.
-
Historical perspective on the synonymization of the four major pest species belonging to the Bactrocera dorsalis species complex (Diptera, Tephritidae).Zookeys. 2015 Nov 26;(540):323-38. doi: 10.3897/zookeys.540.6028. eCollection 2015. Zookeys. 2015. PMID: 26798266 Free PMC article. Review.
Cited by
-
Genome-wide gene expression profiling of the melon fly, Zeugodacus cucurbitae, during thirteen life stages.Sci Data. 2020 Feb 11;7(1):45. doi: 10.1038/s41597-020-0387-9. Sci Data. 2020. PMID: 32047161 Free PMC article.
-
Mechanisms of Novel Host Use by Bactrocera tau (Tephritid: Diptera) Revealed by RNA Transcriptomes.J Insect Sci. 2020 Sep 1;20(5):22. doi: 10.1093/jisesa/ieaa102. J Insect Sci. 2020. PMID: 33078842 Free PMC article.
-
Genome-Wide Analysis of MicroRNAs in Relation to Pupariation in Oriental Fruit Fly.Front Physiol. 2019 Mar 22;10:301. doi: 10.3389/fphys.2019.00301. eCollection 2019. Front Physiol. 2019. PMID: 30967796 Free PMC article.
-
Genomes of the cosmopolitan fruit pest Bactrocera dorsalis (Diptera: Tephritidae) reveal its global invasion history and thermal adaptation.J Adv Res. 2023 Nov;53:61-74. doi: 10.1016/j.jare.2022.12.012. Epub 2022 Dec 24. J Adv Res. 2023. PMID: 36574947 Free PMC article.
-
Genome-wide analysis of long noncoding RNAs and their association in regulating the metamorphosis of the Sarcophaga peregrina (Diptera: Sarcophagidae).PLoS Negl Trop Dis. 2023 Jun 26;17(6):e0011411. doi: 10.1371/journal.pntd.0011411. eCollection 2023 Jun. PLoS Negl Trop Dis. 2023. PMID: 37363930 Free PMC article.
References
-
- Clarke AR, Armstrong KF, Carmichael AE, Milne JR, Raghu S, Roderick GK, Yeates DK. Invasive phytophagous pests arising through a recent tropical evolutionary radiation: the Bactrocera dorsalis complex of fruit flies. Annu Rev Entomol. 2005;50(1):293–319. doi: 10.1146/annurev.ento.50.071803.130428. - DOI - PubMed
-
- Jan Z, Fraenkel G. The mechanism of puparium formation in flies. J Exp Zool Part B. 2010;179(3):315–323.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

