Human chorionic villous mesenchymal stem/stromal cells protect endothelial cells from injury induced by high level of glucose
- PMID: 30241570
- PMCID: PMC6150972
- DOI: 10.1186/s13287-018-0984-0
Human chorionic villous mesenchymal stem/stromal cells protect endothelial cells from injury induced by high level of glucose
Abstract
Background: Mesenchymal stem/stromal cells derived from chorionic villi of human term placentae (pMSCs) protect human endothelial cells from injury induced by hydrogen peroxide (H2O2). In diabetes, elevated levels of glucose (hyperglycaemia) induce H2O2 production, which causes the endothelial dysfunction that underlies the enhanced immune responses and adverse complications associated with diabetes, which leads to thrombosis and atherosclerosis. In this study, we examined the ability of pMSCs to protect endothelial cell functions from the negative impact of high level of glucose.
Methods: pMSCs isolated from the chorionic villi of human term placentae were cultured with endothelial cells isolated from human umbilical cord veins in the presence of glucose. Endothelial cell functions were then determined. The effect of pMSCs on gene expression in glucose-treated endothelial cells was also determined.
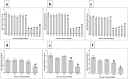
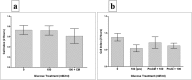
Results: pMSCs reversed the effect of glucose on key endothelial cell functions including proliferation, migration, angiogenesis, and permeability. In addition, pMSCs altered the expression of many genes that mediate important endothelial cell functions including survival, apoptosis, adhesion, permeability, and angiogenesis.
Conclusions: This is the first comprehensive study to provide evidence that pMSCs protect endothelial cells from glucose-induced damage. Therefore, pMSCs have potential therapeutic value as a stem cell-based therapy to repair glucose-induced vascular injury and prevent the adverse complications associated with diabetes and cardiovascular disease. However, further studies are necessary to reveal more detailed aspects of the mechanism of action of pMSCs on glucose-induced endothelial damage in vitro and in vivo.
Keywords: Chorionic villous mesenchymal stromal cells; Endothelial cells; Endothelium permeability; Gene expression; Glucose; Migration; Monocyte invasion; Placenta; Proliferation.
Conflict of interest statement
Ethics approval and consent to participate
The institutional review board (IRB) at King Abdulla International Medical Research Centre (KAIMRC), Saudi Arabia approved this study. Samples (i.e. placentae and umbilical cords) were obtained from uncomplicated human pregnancies (38–40 gestational weeks) following informed patient consent.
Consent for publication
Not applicable. All authors agree to publish this manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures







Similar articles
-
Human decidua basalis mesenchymal stem/stromal cells protect endothelial cell functions from oxidative stress induced by hydrogen peroxide and monocytes.Stem Cell Res Ther. 2018 Oct 25;9(1):275. doi: 10.1186/s13287-018-1021-z. Stem Cell Res Ther. 2018. PMID: 30359307 Free PMC article.
-
Human chorionic villous mesenchymal stem/stromal cells modify the effects of oxidative stress on endothelial cell functions.Placenta. 2017 Nov;59:74-86. doi: 10.1016/j.placenta.2017.05.001. Epub 2017 May 4. Placenta. 2017. PMID: 28502524
-
Phenotypic and functional characterization of mesenchymal stem cells from chorionic villi of human term placenta.Stem Cell Rev Rep. 2013 Feb;9(1):16-31. doi: 10.1007/s12015-012-9385-4. Stem Cell Rev Rep. 2013. PMID: 22628114
-
Significance of Placental Mesenchymal Stem Cell in Placenta Development and Implications for Preeclampsia.Front Pharmacol. 2022 Jun 1;13:896531. doi: 10.3389/fphar.2022.896531. eCollection 2022. Front Pharmacol. 2022. PMID: 35721156 Free PMC article. Review.
-
Adverse effect of high glucose concentration on stem cell therapy.Int J Hematol Oncol Stem Cell Res. 2013;7(3):34-40. Int J Hematol Oncol Stem Cell Res. 2013. PMID: 24505533 Free PMC article. Review.
Cited by
-
Cancer Conditioned Medium Modulates Functional and Phenotypic Properties of Human Decidua Parietalis Mesenchymal Stem/Stromal Cells.Tissue Eng Regen Med. 2019 Nov 1;16(6):615-630. doi: 10.1007/s13770-019-00207-w. eCollection 2019 Dec. Tissue Eng Regen Med. 2019. PMID: 31824824 Free PMC article.
-
Comparative computational analysis to distinguish mesenchymal stem cells from fibroblasts.Front Immunol. 2023 Sep 26;14:1270493. doi: 10.3389/fimmu.2023.1270493. eCollection 2023. Front Immunol. 2023. PMID: 37822926 Free PMC article.
-
Placenta-Derived Mesenchymal Stem Cells (pMSCs) Reverse Diabetes-Associated Endothelial Complications in a Preclinical Animal Model.Int J Mol Sci. 2025 Aug 20;26(16):8057. doi: 10.3390/ijms26168057. Int J Mol Sci. 2025. PMID: 40869378 Free PMC article.
-
Human Placental Mesenchymal Stem/Stromal cells (pMSCs) inhibit agonist-induced platelet functions reducing atherosclerosis and thrombosis phenotypes.J Cell Mol Med. 2021 Oct;25(19):9268-9280. doi: 10.1111/jcmm.16848. Epub 2021 Sep 18. J Cell Mol Med. 2021. PMID: 34535958 Free PMC article.
-
Phenotypic and Functional Responses of Human Decidua Basalis Mesenchymal Stem/Stromal Cells to Lipopolysaccharide of Gram-Negative Bacteria.Stem Cells Cloning. 2021 Nov 2;14:51-69. doi: 10.2147/SCCAA.S332952. eCollection 2021. Stem Cells Cloning. 2021. PMID: 34754198 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

