Paediatric Autism Communication Therapy-Generalised (PACT-G) against treatment as usual for reducing symptom severity in young children with autism spectrum disorder: study protocol for a randomised controlled trial
- PMID: 30241574
- PMCID: PMC6150959
- DOI: 10.1186/s13063-018-2881-3
Paediatric Autism Communication Therapy-Generalised (PACT-G) against treatment as usual for reducing symptom severity in young children with autism spectrum disorder: study protocol for a randomised controlled trial
Abstract
Background: Prior evidence shows that behaviours closely related to the intervention delivered for autism are amenable to change, but it is more difficult to generalise treatment effects beyond the intervention context. We test an early autism intervention designed to promote generalisation of therapy-acquired skills into home and school contexts to improve adaptive function and reduce symptoms. A detailed mechanism study will address the process of such generalisation. Objective 1 will be to test if the PACT-G intervention improves autism symptom outcome in the home and school context of the intervention as well as in the primary outcome research setting. Objective 2 will use the mechanism analysis to test for evidence of acquired skills from intervention generalizing across contexts and producing additive effects on primary outcome.
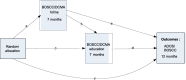
Methods/design: This is a three-site, two-parallel-group, randomised controlled trial of the experimental treatment plus treatment as usual (TAU) versus TAU alone. Children aged 2-11 years (n = 244 (122 intervention/122 TAU; ~ 82/site) meeting criteria for core autism will be eligible. The experimental intervention builds on a clinic-based Pre-school Autism Communication Treatment model (PACT), delivered with the primary caregiver, combined with additional theory- and evidence-based strategies designed to enhance the generalisation of effects into naturalistic home and education contexts. The control intervention will be TAU.
Primary outcome: autism symptom outcome, researcher-assessed using a standardised protocol.
Secondary outcomes: autism symptoms, child interaction with parent or teacher, language and reported functional outcomes in home and school settings. Outcomes measured at baseline and 12-month endpoint in all settings with interim interaction measurements (7 months) to test treatment effect mechanisms. Primary analysis will estimate between-group difference in primary outcome using analysis of covariance with test of homogeneity of effect across age group. Mechanism analysis will use regression models to test for mediation on primary outcome by parent-child and teaching staff-child social interaction.
Discussion: This is an efficacy and mechanism trial of generalising evidence-based autism treatment into home and school settings. It will provide data on whether extending treatment across naturalistic contexts enhances overall effect and data on the mechanism in autism development of the generalisation of acquired developmental skills across contexts.
Trial registration: ISRCTN, ID: 25378536 . Prospectively registered on 9 March 2016.
Keywords: Autism spectrum disorder; Randomised trial; School-based intervention; Social communication intervention.
Conflict of interest statement
Authors’ information
JG and AP are National Institute for Health Research (NIHR) senior investigators. This paper represents independent research part funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Ethics approval and consent to participate
Ethical approval was granted from the North West – Greater Manchester Central Research Ethics Committee on 28 January 2016 (ref: 15/NW/0912).
Consent for publication
Not applicable.
Competing interests
AP declares that he receives royalties from WPS for the Social Communication Questionnaire.
The authors declare registration of a not-for-profit community interest company (number 10902031 INTERACTION METHOD FOR PAEDIATRIC AUTISM COMMUNICATION THERAPY (IMPACT) to deliver/disseminate training on the original PACT intervention method.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- National Institute for Health and Care Excellence. Autism: the management and support of children and young people on the autism spectrum – Clinical guideline 170, 2013. National Institute for Health and Clinical Excellence site. Available at: http://guidance.nice.org.uk/CG170. Accessed 31 Jan 2013.
-
- Green J, Garg S. Annual Research Review: the state of autism intervention science: progress, target psychological and biological mechanisms and future prospects. J Child Psychol Psyc. 2018;59(4) 10.1111/jcpp.12892. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

