Reporting and Interpreting Decision Curve Analysis: A Guide for Investigators
- PMID: 30241973
- PMCID: PMC6261531
- DOI: 10.1016/j.eururo.2018.08.038
Reporting and Interpreting Decision Curve Analysis: A Guide for Investigators
Abstract
Context: Urologists regularly develop clinical risk prediction models to support clinical decisions. In contrast to traditional performance measures, decision curve analysis (DCA) can assess the utility of models for decision making. DCA plots net benefit (NB) at a range of clinically reasonable risk thresholds.
Objective: To provide recommendations on interpreting and reporting DCA when evaluating prediction models.
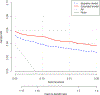
Evidence acquisition: We informally reviewed the urological literature to determine investigators' understanding of DCA. To illustrate, we use data from 3616 patients to develop risk models for high-grade prostate cancer (n=313, 9%) to decide who should undergo a biopsy. The baseline model includes prostate-specific antigen and digital rectal examination; the extended model adds two predictors based on transrectal ultrasound (TRUS).
Evidence synthesis: We explain risk thresholds, NB, default strategies (treat all, treat no one), and test tradeoff. To use DCA, first determine whether a model is superior to all other strategies across the range of reasonable risk thresholds. If so, that model appears to improve decisions irrespective of threshold. Second, consider if there are important extra costs to using the model. If so, obtain the test tradeoff to check whether the increase in NB versus the best other strategy is worth the additional cost. In our case study, addition of TRUS improved NB by 0.0114, equivalent to 1.1 more detected high-grade prostate cancers per 100 patients. Hence, adding TRUS would be worthwhile if we accept subjecting 88 patients to TRUS to find one additional high-grade prostate cancer or, alternatively, subjecting 10 patients to TRUS to avoid one unnecessary biopsy.
Conclusions: The proposed guidelines can help researchers understand DCA and improve application and reporting.
Patient summary: Decision curve analysis can identify risk models that can help us make better clinical decisions. We illustrate appropriate reporting and interpretation of decision curve analysis.
Keywords: Clinical utility; Decision curve analysis; Net benefit; Risk prediction models; Risk threshold; Test tradeoff.
Copyright © 2018 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures
References
-
- Steyerberg EW. Clinical prediction models: a practical approach to development, validation, and updating. New York, NY: Springer; 2009.
-
- Van Calster B, Nieboer D, Vergouwe Y, De Cock B, Pencina MJ, Steyerberg EW. A calibration hierarchy for risk models was defined: from utopia to empirical data. J Clin Epidemiol 2016;74:167–76. - PubMed
-
- Van Calster B, Vickers AJ. Calibration of risk prediction models: impact on decision-analytic performance. Med Decis Making 2015;35:162–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous