Immunotherapy Targeting HPV16/18 Generates Potent Immune Responses in HPV-Associated Head and Neck Cancer
- PMID: 30242022
- PMCID: PMC6320307
- DOI: 10.1158/1078-0432.CCR-18-1763
Immunotherapy Targeting HPV16/18 Generates Potent Immune Responses in HPV-Associated Head and Neck Cancer
Abstract
Purpose: Clinical responses with programmed death (PD-1) receptor-directed antibodies occur in about 20% of patients with advanced head and neck squamous cell cancer (HNSCCa). Viral neoantigens, such as the E6/E7 proteins of HPV16/18, are attractive targets for therapeutic immunization and offer an immune activation strategy that may be complementary to PD-1 inhibition.
Patients and methods: We report phase Ib/II safety, tolerability, and immunogenicity results of immunotherapy with MEDI0457 (DNA immunotherapy targeting HPV16/18 E6/E7 with IL12 encoding plasmids) delivered by electroporation with CELLECTRA constant current device. Twenty-two patients with locally advanced, p16+ HNSCCa received MEDI0457.
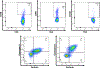
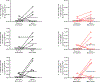
Results: MEDI0457 was associated with mild injection site reactions, but no treatment-related grade 3-5 adverse events (AE) were noted. Eighteen of 21 evaluable patients showed elevated antigen-specific T-cell activity by IFNγ ELISpot, and persistent cellular responses surpassing 100 spot-forming units (SFUs)/106 peripheral blood mononuclear cells (PBMCs) were noted out to 1 year. Induction of HPV-specific CD8+ T cells was observed. MEDI0457 shifted the CD8+/FoxP3+ ratio in 4 of 5 post immunotherapy tumor samples and increased the number of perforin+ immune infiltrates in all 5 patients. One patient developed metastatic disease and was treated with anti-PD-1 therapy with a rapid and durable complete response. Flow-cytometric analyses revealed induction of HPV16-specific PD-1+ CD8+ T cells that were not found prior to MEDI0547 (0% vs. 1.8%).
Conclusions: These data demonstrate that MEDI0457 can generate durable HPV16/18 antigen-specific peripheral and tumor immune responses. This approach may be used as a complementary strategy to PD-1/PD-L1 inhibition in HPV-associated HNSCCa to improve therapeutic outcomes.
©2018 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest Disclosures:Charu Aggarwal reported consulting or advisory roles with Genentech, Bristol-Myers Squibb, Lilly, and Celgene; and institutional research funding from Genetech/Roche, Incyte, Macrogenics, and Merck Sharp & Dohme. Roger B. Cohen reported honoraria from Bristol-Myers Squibb; a consulting or advisory role with Heat Biologics, Takeda, Cerulean Pharma, Kolltan Pharmacueticals, Zymeworks, and Pfizer; institutional research funding from Heat Biologics, Macrogenetics, Merck, Takeda, Cleave Biosciences, and Celldex; and travel, accommodations, or expenses from Heat Biologics, Takeda, Kolltan Pharmaceuticals, Cerulean Pharma, Zymeworks, Bristol-Meyers Squibb, and Pfizer. J.M. Bauml reported consulting or advisory roles with Clovis Oncology, Bristol-Myers Squibb, Merck, AstraZeneca, Genentech, Celgene, Boehringer Ingelheim, and Guardant Health; and institutional research funding from Merck, Carevive Systems, Novartis, Incyte, Bayer, and Janssen. GS Weinstein and Alexander Lin have no relevant disclosures. Russell Vang has no relevant disclosures. David B. Weiner received a SRA research grant from Inovio Pharmaceuticals, has received speakers honoraria from Inovio Pharmaceuticals, GeneOne, Astrazeneca, BMGF, he has ownership interest (including patents) in Inovio Pharmaceuticals and is a consultant/advisory board member for Inovio Pharmaceuticals, a consultant for GeneOne and Astrazeneca and Merck. All authors whose listed association is Inovio Pharmaceuticals (MPM, KAK, AJS, DMK, JB, LS, ST, AA, KD, DM, MD, SO, SD, IC, MLB) and Medimmune (ME, RK) are employees of that entity, own stock or stock options relative to that entity and may own one or more patents relating to the drugs being described in this publication.
Figures








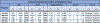

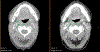
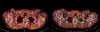
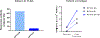
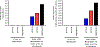
References
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: a cancer journal for clinicians. 2017;67(1):7–30. Epub 2017/01/06. - PubMed
-
- Varilla V, Atienza J, Dasanu CA. Immune alterations and immunotherapy prospects in head and neck cancer. Expert opinion on biological therapy. 2013;13(9):1241–56. Epub 2013/06/25. - PubMed
-
- Sun W, Li WJ, Wu CY, Zhong H, Wen WP. CD45RA-Foxp3high but not CD45RA+Foxp3low suppressive T regulatory cells increased in the peripheral circulation of patients with head and neck squamous cell carcinoma and correlated with tumor progression. Journal of experimental & clinical cancer research : CR. 2014;33:35. Epub 2014/04/26. - PMC - PubMed
-
- Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(32):4294–301. Epub 2011/10/05. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

