Sox14 Is Required for a Specific Subset of Cerebello-Olivary Projections
- PMID: 30242051
- PMCID: PMC6706002
- DOI: 10.1523/JNEUROSCI.1456-18.2018
Sox14 Is Required for a Specific Subset of Cerebello-Olivary Projections
Abstract
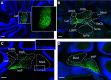
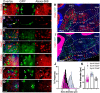
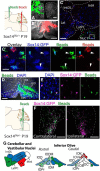
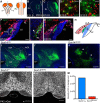
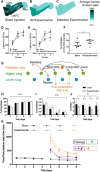
We identify Sox14 as an exclusive marker of inhibitory projection neurons in the lateral and interposed, but not the medial, cerebellar nuclei. Sox14+ neurons make up ∼80% of Gad1+ neurons in these nuclei and are indistinguishable by soma size from other inhibitory neurons. All Sox14+ neurons of the lateral and interposed cerebellar nuclei are generated at approximately E10/10.5 and extend long-range, predominantly contralateral projections to the inferior olive. A small Sox14+ population in the adjacent vestibular nucleus "Y" sends an ipsilateral projection to the oculomotor nucleus. Cerebellar Sox14+ and glutamatergic projection neurons assemble in non-overlapping populations at the nuclear transition zone, and their integration into a coherent nucleus depends on Sox14 function. Targeted ablation of Sox14+ cells by conditional viral expression of diphtheria toxin leads to significantly impaired motor learning. Contrary to expectations, associative learning is unaffected by unilateral Sox14+ neuron elimination in the interposed and lateral nuclei.SIGNIFICANCE STATEMENT The cerebellar nuclei are central to cerebellar function, yet how they modulate and process cerebellar inputs and outputs is still primarily unknown. Our study gives a direct insight into how nucleo-olivary projection neurons are generated, their projections, and their function in an intact behaving mouse. These neurons play a critical conceptual role in all models of cerebellar function, and this study represents the first specific analysis of their molecular identity and function and offers a powerful model for future investigation of cerebellar function in motor control and learning.
Keywords: GABA; axon; cerebellum; genetic model; inferior olive; motor learning.
Copyright © 2018 the authors 0270-6474/18/389539-12$15.00/0.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
