Hypoxia-inducible factor 1-dependent expression of adenosine receptor 2B promotes breast cancer stem cell enrichment
- PMID: 30242135
- PMCID: PMC6187157
- DOI: 10.1073/pnas.1809695115
Hypoxia-inducible factor 1-dependent expression of adenosine receptor 2B promotes breast cancer stem cell enrichment
Erratum in
-
Correction for Lan et al., Hypoxia-inducible factor 1-dependent expression of adenosine receptor 2B promotes breast cancer stem cell enrichment.Proc Natl Acad Sci U S A. 2022 Sep 20;119(38):e2210925119. doi: 10.1073/pnas.2210925119. Epub 2022 Sep 2. Proc Natl Acad Sci U S A. 2022. PMID: 36053742 Free PMC article. No abstract available.
Abstract
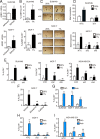
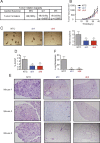
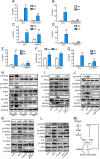
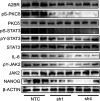
Breast cancer stem cells (BCSCs), which are characterized by a capacity for unlimited self-renewal and for generation of the bulk cancer cell population, play a critical role in cancer relapse and metastasis. Hypoxia is a common feature of the cancer microenvironment that stimulates the specification and maintenance of BCSCs. In this study, we found that hypoxia increased expression of adenosine receptor 2B (A2BR) in human breast cancer cells through the transcriptional activity of hypoxia-inducible factor 1. The binding of adenosine to A2BR promoted BCSC enrichment by activating protein kinase C-δ, which phosphorylated and activated the transcription factor STAT3, leading to increased expression of interleukin 6 and NANOG, two key mediators of the BCSC phenotype. Genetic or pharmacological inhibition of A2BR expression or activity decreased hypoxia- or adenosine-induced BCSC enrichment in vitro, and dramatically impaired tumor initiation and lung metastasis after implantation of MDA-MB-231 human breast cancer cells into the mammary fat pad of immunodeficient mice. These data provide evidence that targeting A2BR might be an effective strategy to eradicate BCSCs.
Keywords: ADORA2B; HIF-1; caffeine; oxygen; tumor-initiating cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Chemotherapy-induced adenosine A2B receptor expression mediates epigenetic regulation of pluripotency factors and promotes breast cancer stemness.Theranostics. 2022 Feb 28;12(6):2598-2612. doi: 10.7150/thno.70581. eCollection 2022. Theranostics. 2022. PMID: 35401817 Free PMC article.
-
Mechanisms of Breast Cancer Stem Cell Specification and Self-Renewal Mediated by Hypoxia-Inducible Factor 1.Stem Cells Transl Med. 2023 Dec 18;12(12):783-790. doi: 10.1093/stcltm/szad061. Stem Cells Transl Med. 2023. PMID: 37768037 Free PMC article.
-
Hypoxia-inducible factors regulate pluripotency factor expression by ZNF217- and ALKBH5-mediated modulation of RNA methylation in breast cancer cells.Oncotarget. 2016 Oct 4;7(40):64527-64542. doi: 10.18632/oncotarget.11743. Oncotarget. 2016. PMID: 27590511 Free PMC article.
-
Hypoxia-inducible factors promote breast cancer stem cell specification and maintenance in response to hypoxia or cytotoxic chemotherapy.Adv Cancer Res. 2019;141:175-212. doi: 10.1016/bs.acr.2018.11.001. Epub 2018 Dec 19. Adv Cancer Res. 2019. PMID: 30691683 Review.
-
Regulation of the breast cancer stem cell phenotype by hypoxia-inducible factors.Clin Sci (Lond). 2015 Dec;129(12):1037-45. doi: 10.1042/CS20150451. Clin Sci (Lond). 2015. PMID: 26405042 Review.
Cited by
-
The elegant complexity of mammalian ecto-5'-nucleotidase (CD73).Trends Cell Biol. 2021 Oct;31(10):829-842. doi: 10.1016/j.tcb.2021.05.008. Epub 2021 Jun 8. Trends Cell Biol. 2021. PMID: 34116887 Free PMC article. Review.
-
Expression of Adenosine Receptors in Rodent Pancreas.Int J Mol Sci. 2019 Oct 25;20(21):5329. doi: 10.3390/ijms20215329. Int J Mol Sci. 2019. PMID: 31717704 Free PMC article.
-
Augmentation of Extracellular ATP Synergizes With Chemotherapy in Triple Negative Breast Cancer.Front Oncol. 2022 Apr 20;12:855032. doi: 10.3389/fonc.2022.855032. eCollection 2022. Front Oncol. 2022. PMID: 35515134 Free PMC article.
-
Role of the Microenvironment in Regulating Normal and Cancer Stem Cell Activity: Implications for Breast Cancer Progression and Therapy Response.Cancers (Basel). 2019 Aug 24;11(9):1240. doi: 10.3390/cancers11091240. Cancers (Basel). 2019. PMID: 31450577 Free PMC article. Review.
-
The metabolic flexibility of quiescent CSC: implications for chemotherapy resistance.Cell Death Dis. 2021 Sep 4;12(9):835. doi: 10.1038/s41419-021-04116-6. Cell Death Dis. 2021. PMID: 34482364 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A, Cancer statistics, 2017. CA Cancer J Clin 67, 7–30 (2017). - PubMed
-
- Ponti D, et al. , Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res 65, 5506–5511 (2005). - PubMed
-
- Al-Ejeh F, et al. , Breast cancer stem cells: Treatment resistance and therapeutic opportunities. Carcinogenesis 32, 650–658 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

