Notch ligand Jagged1 promotes mesenchymal stromal cell-based cartilage repair
- PMID: 30242147
- PMCID: PMC6155067
- DOI: 10.1038/s12276-018-0151-9
Notch ligand Jagged1 promotes mesenchymal stromal cell-based cartilage repair
Abstract
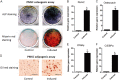
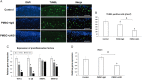
Placenta-derived mesenchymal stromal cells (PMSCs) provide a promising cell source for tissue regeneration. However, rapid induction of PMSC chondrogenic differentiation during therapeutic transplantation remains extremely challenging. Here we undertook a study to determine if Notch inhibition by soluble Jagged1 (JAG1) peptides could be utilized to accelerate PMSC-induced cartilage regeneration in a mouse post-traumatic osteoarthritis (PTOA) model. Our results showed that treatment of PMSCs with soluble JAG1 significantly enhanced chondrogenesis in culture as shown by increased alcian blue staining and decreased Notch target Hes1 expression when compared to those in lgG-treated control cells. Importantly, significantly enhanced cartilage formation and decreased joint inflammation were observed when JAG1-treated PMSCs were injected into mouse PTOA knee joints. Finally, in vivo cell tracing showed that more JAG1-treated PMSCs remained in knee joint tissues and that JAG1-treated PMSCs exhibited greater PMSC chondrogenic differentiation than lgG-treated control PMSCs at 4 weeks after injection. These data indicate that transient Notch inhibition by soluble JAG1 could be used to enhance PMSC survival and chondrogenic differentiation, thereby increasing the therapeutic potential of PMSCs for cartilage regeneration.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

