A tissue-specific self-interacting chromatin domain forms independently of enhancer-promoter interactions
- PMID: 30242161
- PMCID: PMC6155075
- DOI: 10.1038/s41467-018-06248-4
A tissue-specific self-interacting chromatin domain forms independently of enhancer-promoter interactions
Abstract
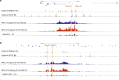
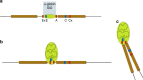
Self-interacting chromatin domains encompass genes and their cis-regulatory elements; however, the three-dimensional form a domain takes, whether this relies on enhancer-promoter interactions, and the processes necessary to mediate the formation and maintenance of such domains, remain unclear. To examine these questions, here we use a combination of high-resolution chromosome conformation capture, a non-denaturing form of fluorescence in situ hybridisation and super-resolution imaging to study a 70 kb domain encompassing the mouse α-globin regulatory locus. We show that this region forms an erythroid-specific, decompacted, self-interacting domain, delimited by frequently apposed CTCF/cohesin binding sites early in terminal erythroid differentiation, and does not require transcriptional elongation for maintenance of the domain structure. Formation of this domain does not rely on interactions between the α-globin genes and their major enhancers, suggesting a transcription-independent mechanism for establishment of the domain. However, absence of the major enhancers does alter internal domain interactions. Formation of a loop domain therefore appears to be a mechanistic process that occurs irrespective of the specific interactions within.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
- MC_UU_12009/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12009/15/MRC_/Medical Research Council/United Kingdom
- MC_UU_00016/1/MRC_/Medical Research Council/United Kingdom
- MC_U137961145/MRC_/Medical Research Council/United Kingdom
- 106130/Z/14/Z/WT_/Wellcome Trust/United Kingdom
- MC_U137961144/MRC_/Medical Research Council/United Kingdom
- MC_UU_00016/14/MRC_/Medical Research Council/United Kingdom
- MC_UU_00016/4/MRC_/Medical Research Council/United Kingdom
- MC_PC_15069/MRC_/Medical Research Council/United Kingdom
- G1000801/MRC_/Medical Research Council/United Kingdom
- MR/N00969X/1/MRC_/Medical Research Council/United Kingdom
- MR/S005382/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12009/4/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

