Two-photon microscopy of Paneth cells in the small intestine of live mice
- PMID: 30242205
- PMCID: PMC6155010
- DOI: 10.1038/s41598-018-32640-7
Two-photon microscopy of Paneth cells in the small intestine of live mice
Abstract
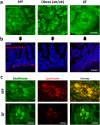
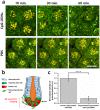
Paneth cells are one of the principal epithelial cell types in the small intestine, located at the base of intestinal crypts. Paneth cells play key roles in intestinal host-microbe homeostasis via granule secretion, and their dysfunction is implicated in pathogenesis of several diseases including Crohn's disease. Despite their physiological importance, study of Paneth cells has been hampered by the limited accessibility and lack of labeling methods. In this study, we developed a simple in vivo imaging method of Paneth cells in the intact mouse small intestine by using moxifloxacin and two-photon microscopy (TPM). Moxifloxacin, an FDA-approved antibiotic, was used for labeling cells and its fluorescence was strongly observed in Paneth cell granules by TPM. Moxifloxacin labeling of Paneth cell granules was confirmed by molecular counterstaining. Comparison of Paneth cells in wild type, genetically obese (ob/ob), and germ-free (GF) mice showed different granule distribution. Furthermore, Paneth cell degranulation was observed in vivo. Our study suggests that TPM with moxifloxacin labeling can serve as a useful tool for studying Paneth cell biology and related diseases.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- 2011-0019633/National Research Foundation of Korea (NRF)/International
- 2011-0030075/National Research Foundation of Korea (NRF)/International
- 2011-0019633/National Research Foundation of Korea (NRF)/International
- 2011-0030075/National Research Foundation of Korea (NRF)/International
- 2011-0019633/National Research Foundation of Korea (NRF)/International
- 2011-0030075/National Research Foundation of Korea (NRF)/International
- 2011-0030075/National Research Foundation of Korea (NRF)/International
- 10Z20130012243/Ministry of Education (Ministry of Education of the Republic of Korea)/International
- 10Z20130012243/Ministry of Education (Ministry of Education of the Republic of Korea)/International
- 10Z20130012243/Ministry of Education (Ministry of Education of the Republic of Korea)/International
- 10Z20130012243/Ministry of Education (Ministry of Education of the Republic of Korea)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

