In-vivo shift of the microbiota in oral biofilm in response to frequent sucrose consumption
- PMID: 30242260
- PMCID: PMC6155074
- DOI: 10.1038/s41598-018-32544-6
In-vivo shift of the microbiota in oral biofilm in response to frequent sucrose consumption
Abstract
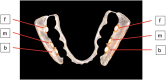
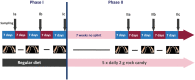
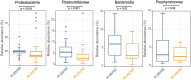
Caries is associated with shifts of microbiota in dental biofilms and primarily driven by frequent sucrose consumption. Data on environmentally induced in vivo microbiota shifts are scarce therefore we investigated the influence of frequent sucrose consumption on the oral biofilm. Splint systems containing enamel slabs were worn for 3 × 7 days with 7-day intervals to obtain oral biofilm samples. After a three-month dietary change of sucking 10 g of sucrose per day in addition to the regular diet, biofilm was obtained again at the end of the second phase. The microbiota was analysed using Illumina MiSeq amplicon sequencing (v1-v2 region). In addition, roughness of the enamel surface was measured with laser scanning microscopy. The sucrose phase resulted in significant differences in beta-diversity and significantly decreased species richness. It was marked by a significant increase in abundance of streptococci, specifically Streptococcus gordonii, Streptococcus parasanguinis and Streptococcus sanguinis. Enamel surface roughness began to increase, reflecting initial impairment of dental enamel surface. The results showed that frequent sucrose consumption provoked compositional changes in the microbiota, leading to an increase of non-mutans streptococci, hence supporting the extended ecological plaque hypothesis and emphasizing the synergy of multiple bacterial species in the development of caries.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

