Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis
- PMID: 30242316
- PMCID: PMC6440712
- DOI: 10.1001/jamaoncol.2018.3923
Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis
Erratum in
-
Error in Figure 1.JAMA Oncol. 2018 Dec 1;4(12):1792. doi: 10.1001/jamaoncol.2018.5346. JAMA Oncol. 2018. PMID: 30325999 Free PMC article. No abstract available.
Abstract
Importance: Immune checkpoint inhibitors (ICIs) are now a mainstay of cancer treatment. Although rare, fulminant and fatal toxic effects may complicate these otherwise transformative therapies; characterizing these events requires integration of global data.
Objective: To determine the spectrum, timing, and clinical features of fatal ICI-associated toxic effects.
Design, setting, and participants: We retrospectively queried a World Health Organization (WHO) pharmacovigilance database (Vigilyze) comprising more than 16 000 000 adverse drug reactions, and records from 7 academic centers. We performed a meta-analysis of published trials of anti-programmed death-1/ligand-1 (PD-1/PD-L1) and anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) to evaluate their incidence using data from large academic medical centers, global WHO pharmacovigilance data, and all published ICI clinical trials of patients with cancer treated with ICIs internationally.
Exposures: Anti-CTLA-4 (ipilimumab or tremelimumab), anti-PD-1 (nivolumab, pembrolizumab), or anti-PD-L1 (atezolizumab, avelumab, durvalumab).
Main outcomes and measures: Timing, spectrum, outcomes, and incidence of ICI-associated toxic effects.
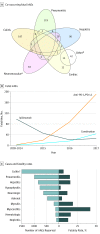
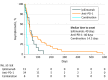
Results: Internationally, 613 fatal ICI toxic events were reported from 2009 through January 2018 in Vigilyze. The spectrum differed widely between regimens: in a total of 193 anti-CTLA-4 deaths, most were usually from colitis (135 [70%]), whereas anti-PD-1/PD-L1-related fatalities were often from pneumonitis (333 [35%]), hepatitis (115 [22%]), and neurotoxic effects (50 [15%]). Combination PD-1/CTLA-4 deaths were frequently from colitis (32 [37%]) and myocarditis (22 [25%]). Fatal toxic effects typically occurred early after therapy initiation for combination therapy, anti-PD-1, and ipilimumab monotherapy (median 14.5, 40, and 40 days, respectively). Myocarditis had the highest fatality rate (52 [39.7%] of 131 reported cases), whereas endocrine events and colitis had only 2% to 5% reported fatalities; 10% to 17% of other organ-system toxic effects reported had fatal outcomes. Retrospective review of 3545 patients treated with ICIs from 7 academic centers revealed 0.6% fatality rates; cardiac and neurologic events were especially prominent (43%). Median time from symptom onset to death was 32 days. A meta-analysis of 112 trials involving 19 217 patients showed toxicity-related fatality rates of 0.36% (anti-PD-1), 0.38% (anti-PD-L1), 1.08% (anti-CTLA-4), and 1.23% (PD-1/PD-L1 plus CTLA-4).
Conclusions and relevance: In the largest evaluation of fatal ICI-associated toxic effects published to date to our knowledge, we observed early onset of death with varied causes and frequencies depending on therapeutic regimen. Clinicians across disciplines should be aware of these uncommon lethal complications.
Conflict of interest statement
Figures
Comment in
-
Insights into the risk of fatal AEs with ICIs.Nat Rev Clin Oncol. 2018 Nov;15(11):653. doi: 10.1038/s41571-018-0104-1. Nat Rev Clin Oncol. 2018. PMID: 30262917 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

