Small-Molecule Inhibitors of PARPs: From Tools for Investigating ADP-Ribosylation to Therapeutics
- PMID: 30242511
- PMCID: PMC11793873
- DOI: 10.1007/82_2018_137
Small-Molecule Inhibitors of PARPs: From Tools for Investigating ADP-Ribosylation to Therapeutics
Abstract
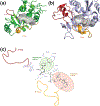
Over the last 60 years, poly-ADP-ribose polymerases (PARPs, 17 family members in humans) have emerged as important regulators of physiology and disease. Small-molecule inhibitors have been essential tools for unraveling PARP function, and recently the first PARP inhibitors have been approved for the treatment of various human cancers. However, inhibitors have only been developed for a few PARPs and in vitro profiling has revealed that many of these exhibit polypharmacology across the PARP family. In this review, we discuss the history, development, and current state of the field, highlighting the limitations and opportunities for PARP inhibitor development.
Figures




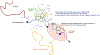


Similar articles
-
Enabling drug discovery for the PARP protein family through the detection of mono-ADP-ribosylation.Biochem Pharmacol. 2019 Sep;167:97-106. doi: 10.1016/j.bcp.2019.05.007. Epub 2019 May 7. Biochem Pharmacol. 2019. PMID: 31075269
-
Host poly(ADP-ribose) polymerases (PARPs) in acute and chronic bacterial infections.Microbes Infect. 2019 Dec;21(10):423-431. doi: 10.1016/j.micinf.2019.06.002. Epub 2019 Jun 15. Microbes Infect. 2019. PMID: 31207286 Review.
-
Chemical genetic methodologies for identifying protein substrates of PARPs.Trends Biochem Sci. 2022 May;47(5):390-402. doi: 10.1016/j.tibs.2021.07.002. Epub 2021 Aug 5. Trends Biochem Sci. 2022. PMID: 34366182 Review.
-
Poly (ADP-Ribose) Polymerases (PARPs) and PARP Inhibitor-Targeted Therapeutics.Anticancer Agents Med Chem. 2019;19(2):206-212. doi: 10.2174/1871520618666181109164645. Anticancer Agents Med Chem. 2019. PMID: 30417796 Review.
-
PARP enzymes and mono-ADP-ribosylation: advancing the connection from interferon-signalling to cancer biology.Expert Rev Mol Med. 2024 Aug 27;26:e17. doi: 10.1017/erm.2024.13. Expert Rev Mol Med. 2024. PMID: 39189367 Free PMC article. Review.
Cited by
-
PASTA: PARP activity screening and inhibitor testing assay.STAR Protoc. 2021 Feb 18;2(1):100344. doi: 10.1016/j.xpro.2021.100344. eCollection 2021 Mar 19. STAR Protoc. 2021. PMID: 33665624 Free PMC article.
-
ADP-Ribosylation as Post-Translational Modification of Proteins: Use of Inhibitors in Cancer Control.Int J Mol Sci. 2021 Oct 7;22(19):10829. doi: 10.3390/ijms221910829. Int J Mol Sci. 2021. PMID: 34639169 Free PMC article. Review.
-
Rational design of selective inhibitors of PARP4.RSC Med Chem. 2021 Oct 4;12(11):1950-1957. doi: 10.1039/d1md00195g. eCollection 2021 Nov 17. RSC Med Chem. 2021. PMID: 34825190 Free PMC article.
-
Integrating Cancer Vaccines in the Standard-of-Care of Ovarian Cancer: Translating Preclinical Models to Human.Cancers (Basel). 2021 Sep 10;13(18):4553. doi: 10.3390/cancers13184553. Cancers (Basel). 2021. PMID: 34572778 Free PMC article. Review.
-
Targeted Drug Delivery in Periorbital Non-Melanocytic Skin Malignancies.Bioengineering (Basel). 2024 Oct 15;11(10):1029. doi: 10.3390/bioengineering11101029. Bioengineering (Basel). 2024. PMID: 39451404 Free PMC article. Review.
References
-
- Andersson CD, Karlberg T, Ekblad T, Lindgren AEG, Thorsell A-G, Spjut S et al. (2012) Discovery of ligands for ADP-ribosyltransferases via docking-based virtual screening. J Med Chem 55(17):7706–7718 - PubMed
-
- Andreone TL, O’Connor M, Denenberg A, Hake PW, Zingarelli B (2003) Poly(ADP-Ribose) polymerase-1 regulates activation of activator protein-1 in murine fibroblasts. J Immunol 170 (4):2113–2120. 15 Feb 2003. (American Association of Immunologists; ) - PubMed
-
- Bai P (2015) Biology of poly(ADP-Ribose) polymerases: the factotums of cell maintenance. Mol Cell 58(6):947–958 - PubMed
-
- Bai P, Virág L (2012) Role of poly(ADP-ribose) polymerases in the regulation of inflammatory processes. FEBS Lett 586(21):3771–3777. 26 Sep 2012. (Wiley-Blackwell; ) - PubMed
-
- Barbarulo A, Iansante V, Chaidos A, Naresh K, Rahemtulla A, Franzoso G et al. (2013) Poly (ADP-ribose) polymerase family member 14 (PARP14) is a novel effector of the JNK2-dependent pro-survival signal in multiple myeloma. Oncogene 32(36):4231–4242 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

