Coherent phenomena in photosynthetic light harvesting: part two-observations in biological systems
- PMID: 30242555
- PMCID: PMC6233342
- DOI: 10.1007/s12551-018-0456-x
Coherent phenomena in photosynthetic light harvesting: part two-observations in biological systems
Abstract
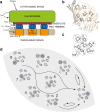
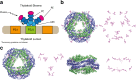
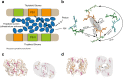
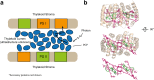
Considerable debate surrounds the question of whether or not quantum mechanics plays a significant, non-trivial role in photosynthetic light harvesting. Many have proposed that quantum superpositions and/or quantum transport phenomena may be responsible for the efficiency and robustness of energy transport present in biological systems. The critical experimental observations comprise the observation of coherent oscillations or "quantum beats" via femtosecond laser spectroscopy, which have been observed in many different light harvesting systems. Part Two of this review aims to provide an overview of experimental observations of energy transfer in the most studied light harvesting systems. Length scales, derived from crystallographic studies, are combined with energy and time scales of the beats observed via spectroscopy. A consensus is emerging that most long-lived (hundreds of femtoseconds) coherent phenomena are of vibrational or vibronic origin, where the latter may result in coherent excitation transport within a protein complex. In contrast, energy transport between proteins is likely to be incoherent in nature. The question of whether evolution has selected for these non-trivial quantum phenomena may be an unanswerable question, as dense packings of chromophores will lead to strong coupling and hence non-trivial quantum phenomena. As such, one cannot discern whether evolution has optimised light harvesting systems for high chromophore density or for the ensuing quantum effects as these are inextricably linked and cannot be switched off.
Keywords: Light harvesting; Photosynthesis; Protein; Quantum biology; Quantum coherence.
Conflict of interest statement
Conflict of interest
Harry W. Rathbone declares that he has no conflict of interest. Jeffery A. Davis declares that he has no conflict of interest. Katharine A. Michie declares that she has no conflict of interest. Sophia C. Goodchild declares that she has no conflict of interest. Neil O. Robertson declares that he has no conflict of interest. Paul M.G. Curmi declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Figures


References
-
- Arpin PC, Turner DB, McClure SD, et al. Spectroscopic studies of cryptophyte light harvesting proteins: vibrations and coherent oscillations. J Phys Chem B. 2015;119:10025–10034. - PubMed
-
- Baghbanzadeh S, Kassal I. Geometry, supertransfer, and optimality in the light harvesting of purple bacteria. J Phys Chem Lett. 2016;7:3804–3811. - PubMed
-
- Book LD, Ostafin AE, Ponomarenko N, et al. Exciton delocalization and initial dephasing dynamics of purple bacterial LH2. J Phys Chem B. 2000;104:8295–8307.
-
- Bricker WP, Lo CS. Efficient pathways of excitation energy transfer from delocalized S2 excitons in the peridinin-chlorophyll a-protein complex. J Phys Chem B. 2015;119:5755–5764. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

