A PRISMA-compliant systematic review of the endpoints employed to evaluate symptomatic treatments for primary headaches
- PMID: 30242571
- PMCID: PMC6742919
- DOI: 10.1186/s10194-018-0920-9
A PRISMA-compliant systematic review of the endpoints employed to evaluate symptomatic treatments for primary headaches
Erratum in
-
Correction to: A PRISMA-compliant systematic review of the endpoints employed to evaluate symptomatic treatments for primary headaches.J Headache Pain. 2019 Feb 13;20(1):14. doi: 10.1186/s10194-019-0967-2. J Headache Pain. 2019. PMID: 30760196 Free PMC article.
Abstract
Background: Primary headache are prevalent and debilitating disorders. Acute pain cessation is one of the key points in their treatment. Many drugs have been studied but the design of the trials is not usually homogeneous. Efficacy of the trial is determined depending on the selected primary endpoint and usually other different outcomes are measured. We aim to critically appraise which were the employed outcomes through a systematic review.
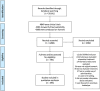
Methods: We conducted a systematic review of literature focusing on studies on primary headache evaluating acute relief of pain, following the PRISMA guideline. The study population included patients participating in a controlled study about symptomatic treatment. The comparator could be placebo or the standard of care. The collected information was the primary outcome of the study and all secondary outcomes. We evaluated the studied drug, the year of publication and the type of journal. We performed a search and we screened all the potential papers and reviewed them considering inclusion/exclusion criteria.
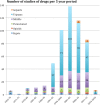
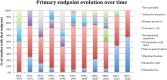
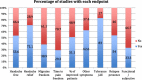
Results: The search showed 4288 clinical trials that were screened and 794 full articles were assessed for eligibility for a final inclusion of 495 papers. The studies were published in headache specific journals (58%), general journals (21.6%) and neuroscience journals (20.4%). Migraine was the most studied headache, in 87.8% studies, followed by tension type headache in 4.7%. Regarding the most evaluated drug, triptans represented 68.6% of all studies, followed by non-steroidal anti-inflammatories (25.1%). Only 4.6% of the papers evaluated ergots and 1.6% analyzed opioids. The most frequent primary endpoint was the relief of the headache at a determinate moment, in 54.1%. Primary endpoint was evaluated at 2-h in 69.9% of the studies. Concerning other endpoints, tolerance was the most frequently addressed (83%), followed by headache relief (71.1%), improvement of other symptoms (62.5%) and presence of relapse (54%). The number of secondary endpoints increased from 4.2 (SD = 2.0) before 1991 to 6.39 after 2013 (p = 0.001).
Conclusion: Headache relief has been the most employed primary endpoint but headache disappearance starts to be firmly considered. The number of secondary endpoints increases over time and other outcomes such as disability, quality of life and patients' preference are receiving attention.
Keywords: Acute; Clinical trials; Endpoints; Non-steroidal anti-inflammatory; Primary headaches; Prisma-guidelines; Triptans.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2015;388:1545–602 - PMC - PubMed
-
- Committee on Clinical Trials in Migraine. Guidelines for Controlled Trials of Drugs in Migraine. First ed Cephalalgia 1991;11(1):1-12 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

