Efficacy of pregabalin in post-traumatic peripheral neuropathic pain: a randomized, double-blind, placebo-controlled phase 3 trial
- PMID: 30242745
- PMCID: PMC6244661
- DOI: 10.1007/s00415-018-9063-9
Efficacy of pregabalin in post-traumatic peripheral neuropathic pain: a randomized, double-blind, placebo-controlled phase 3 trial
Abstract
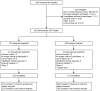
The growing need for symptomatic treatment of post-traumatic neuropathic pain (PTNP) continues to be unmet. Studies evaluating the efficacy of pregabalin for reducing neuropathic pain following trauma and surgery yielded positive results over ≤ 8-week treatment. To assess the efficacy and tolerability of pregabalin over 3 months in patients with PTNP, a randomized, double-blind, placebo-controlled, parallel-group trial evaluated patients with PTNP at 101 centers in 11 countries-the longest, largest such trial. Adults diagnosed with PTNP were randomly assigned (1:1) to 15 weeks of pregabalin (flexibly dosed 150-600 mg/day) or matching placebo. Primary efficacy analysis was by mixed-model repeated measures comparing change from baseline to week 15 in weekly mean pain scores between active and placebo groups. Evaluable patients included 274 in the pregabalin group and 265 in the placebo group. Trauma was surgical in 49.6% of patients, non-surgical in the remainder. The primary efficacy analysis showed no statistically significant difference between pregabalin and placebo groups in the change from baseline to week 15 [mean difference, - 0.22 points (95% confidence interval, 0.54-0.10); p = 0.1823]. However, comparisons for key secondary outcome measures yielded p values < 0.05 favoring pregabalin. Consistent with the known safety profile of pregabalin, the most common adverse events were dizziness and somnolence (14.6 and 9.9% of patients, respectively) with pregabalin (vs 4.2 and 3.4% with placebo). These findings demonstrate the feasibility of conducting a large, phase 3 registration trial in the heterogeneous PTNP study population.ClinicalTrials.gov NCT01701362.
Keywords: Neuropathic pain; Post-surgical neuropathic pain; Post-traumatic neuropathic pain; Pregabalin.
Conflict of interest statement
Ethical standards
The protocol complied with the Declaration of Helsinki (1964), and was reviewed and approved by the institutional review board at each participating center.
Informed consent
All participants provided written, informed consent.
Conflicts of interest
JM has participated in advisory boards (Pfizer, Editas Medicine, Flexion Therapeutics, Teva, Quark, Pacira, Inspirion Delivery Sciences, Quartet, Pacira Egalet, Biogen, Nektar, Endo, Immune Pharma, Chromocell, Collegium, Purdue, Novartis, Sanofi, Convergence, Aptinyx, Daiichi Sankyo, Allergan, Plasmasurgical, and Grunenthal), received research funding (Depomed, Pfizer), and served on Data Safety Monitoring Boards (Allergan, Novartis). M Resnick, R Yang, J Scavone, E Whalen, G Gregorian, B Parsons, and L Knapp are employees of Pfizer Inc. S Greenberg was an employee of Pfizer at the time of the study and development of the manuscript. N Katz is employed by Analgesic Solutions, which provided a central eligibility verification service to identify patients with PTNP.
Data sharing statement
Upon request, and subject to certain criteria, conditions and exceptions (see
Figures

References
-
- Haanpaa M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D, Cruccu G, Hansson P, Haythornthwaite JA, Iannetti GD, Jensen TS, Kauppila T, Nurmikko TJ, Rice AS, Rowbotham M, Serra J, Sommer C, Smith BH, Treede RD. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152(1):14–27. doi: 10.1016/j.pain.2010.07.031. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

