Certolizumab pegol for the treatment of patients with moderate-to-severe chronic plaque psoriasis: pooled analysis of week 16 data from three randomized controlled trials
- PMID: 30242918
- PMCID: PMC6646900
- DOI: 10.1111/jdv.15258
Certolizumab pegol for the treatment of patients with moderate-to-severe chronic plaque psoriasis: pooled analysis of week 16 data from three randomized controlled trials
Abstract
Background: Certolizumab pegol, an Fc-free, PEGylated, anti-tumour necrosis factor (TNF) biologic, has demonstrated favourable results in three ongoing, phase 3, randomized, double-blinded, placebo-controlled trials in adults with psoriasis.
Objective: Data were pooled from the ongoing trials to investigate efficacy in selected subgroups and add precision to estimates of treatment effects during the initial 16 weeks of treatment.
Methods: In each trial, patients ≥18 years with moderate-to-severe chronic plaque psoriasis for ≥6 months were randomized to receive certolizumab 400 mg, certolizumab 200 mg or placebo every 2 weeks for 16 weeks. Coprimary endpoints for the pooled analysis were responder rates at Week 16, defined as ≥75% reduction in psoriasis area and severity index (PASI 75) and physician global assessment (PGA) of 0/1 ('clear'/'almost clear' with ≥2-category improvement). Safety was assessed by treatment-emergent adverse events.
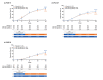
Results: A total of 850 patients treated with certolizumab 400 mg (N = 342), certolizumab 200 mg (N = 351) or placebo (N = 157) were included in the pooled analysis. At Week 16, PASI 75 and PGA 0/1 responder rates were 80.1% and 63.7% in the certolizumab 400 mg group, 74.5% and 54.6% in the certolizumab 200 mg group, and 7.5% and 2.8% in the placebo group (P < 0.0001 for each dose versus placebo). In patients with and without prior biologic therapy, both doses of certolizumab resulted in substantially higher responder rates versus placebo. The incidence of adverse events was generally similar between the 400 mg and placebo groups, and somewhat lower in the 200 mg group versus placebo. No new safety signals were identified.
Conclusion: Certolizumab pegol 400 mg or 200 mg every 2 weeks for 16 weeks was associated with statistically significant and clinically meaningful improvements in signs and symptoms of psoriasis in patients with and without prior biologic therapy, and a safety profile consistent with the anti-TNF class in psoriasis.
© 2018 European Academy of Dermatology and Venereology.
Figures

References
-
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol 2014; 70: 512–516. - PubMed
-
- Danielsen K, Olsen AO, Wilsgaard T, Furberg AS. Is the prevalence of psoriasis increasing? A 30‐year follow‐up of a population‐based cohort. Br J Dermatol 2013; 168: 1303–1310. - PubMed
-
- Gniadecki R, Bang B, Bryld LE, Iversen L, Lasthein S, Skov L. Comparison of long‐term drug survival and safety of biologic agents in patients with psoriasis vulgaris. Br J Dermatol 2015; 172: 244–252. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

