L-plastin phosphorylation regulates the early phase of sealing ring formation by actin bundling process in mouse osteoclasts
- PMID: 30244178
- PMCID: PMC6181588
- DOI: 10.1016/j.yexcr.2018.09.014
L-plastin phosphorylation regulates the early phase of sealing ring formation by actin bundling process in mouse osteoclasts
Abstract
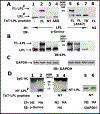
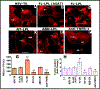
The process of sealing ring formation requires major actin filament reorganization. We previously demonstrated that an actin-bundling protein L-plastin has a role in the cross-linking of actin filaments into tight bundles and forms actin aggregates (denoted as nascent sealing zones). These nascent sealing zones mature into fully functional sealing rings. We have shown here that TNF-alpha signaling regulates the phosphorylation of serine-5 and -7 in L-plastin which increases the actin bundling capacity of L-plastin and hence the formation of nascent sealing zones in mouse osteoclasts. Using the TAT-mediated transduction method, we confirmed the role of L-plastin in nascent sealing zones formation at the early phase of the sealing ring assembly. Transduction of TAT-fused full-length L-plastin peptide significantly increases the number of nascent sealing zones and therefore sealing rings. But, transduction of amino-terminal L-plastin peptides consisting of the serine-5 and -7 reduces the formation of both nascent sealing zones and sealing rings. Therefore, bone resorption in vitro was reduced considerably. The decrease was associated with the selective inhibition of cellular L-plastin phosphorylation by the transduced peptides. Neither the formation of podosomes nor the migration was affected in these osteoclasts. Phosphorylation of L- plastin on serine 5 and -7 residues increases the F-actin bundling capacity. The significance of our studies stands on laying the groundwork for a better understanding of L-plastin as a potential regulator at the early phase of sealing ring formation and could be a new therapeutic target to treat bone loss.
Keywords: Bone resorption; Cytoskeleton; L-Plastin; Nascent sealing zones; Osteoclasts; Sealing rings.
Published by Elsevier Inc.
Conflict of interest statement
Disclosures
All authors declare that they have no conflicts of interest.
Figures






Similar articles
-
L-Plastin Phosphorylation: Possible Regulation by a TNFR1 Signaling Cascade in Osteoclasts.Cells. 2021 Sep 15;10(9):2432. doi: 10.3390/cells10092432. Cells. 2021. PMID: 34572081 Free PMC article.
-
Peptidomimetic inhibitors of L-plastin reduce the resorptive activity of osteoclast but not the bone forming activity of osteoblasts in vitro.PLoS One. 2018 Sep 24;13(9):e0204209. doi: 10.1371/journal.pone.0204209. eCollection 2018. PLoS One. 2018. PMID: 30248139 Free PMC article.
-
Regulation of sealing ring formation by L-plastin and cortactin in osteoclasts.J Biol Chem. 2010 Sep 24;285(39):29911-24. doi: 10.1074/jbc.M109.099697. Epub 2010 Jul 22. J Biol Chem. 2010. PMID: 20650888 Free PMC article.
-
Podosome and sealing zone: specificity of the osteoclast model.Eur J Cell Biol. 2006 Apr;85(3-4):195-202. doi: 10.1016/j.ejcb.2005.09.008. Epub 2005 Oct 24. Eur J Cell Biol. 2006. PMID: 16546562 Review.
-
The Sealing Zone in Osteoclasts: A Self-Organized Structure on the Bone.Int J Mol Sci. 2018 Mar 26;19(4):984. doi: 10.3390/ijms19040984. Int J Mol Sci. 2018. PMID: 29587415 Free PMC article. Review.
Cited by
-
New Proteins Contributing to Immune Cell Infiltration and Pannus Formation of Synovial Membrane from Arthritis Diseases.Int J Mol Sci. 2021 Dec 31;23(1):434. doi: 10.3390/ijms23010434. Int J Mol Sci. 2021. PMID: 35008858 Free PMC article.
-
L-Plastin Phosphorylation: Possible Regulation by a TNFR1 Signaling Cascade in Osteoclasts.Cells. 2021 Sep 15;10(9):2432. doi: 10.3390/cells10092432. Cells. 2021. PMID: 34572081 Free PMC article.
-
Peptidomimetic inhibitor of L-plastin reduces osteoclastic bone resorption in aging female mice.Bone Res. 2021 Apr 9;9(1):22. doi: 10.1038/s41413-020-00135-9. Bone Res. 2021. PMID: 33837180 Free PMC article.
-
Targeting actin-bundling protein L-plastin as an anabolic therapy for bone loss.Sci Adv. 2020 Nov 18;6(47):eabb7135. doi: 10.1126/sciadv.abb7135. Print 2020 Nov. Sci Adv. 2020. PMID: 33208358 Free PMC article.
-
Engineering of L-Plastin Peptide-Loaded Biodegradable Nanoparticles for Sustained Delivery and Suppression of Osteoclast Function In Vitro.Int J Cell Biol. 2019 May 5;2019:6943986. doi: 10.1155/2019/6943986. eCollection 2019. Int J Cell Biol. 2019. PMID: 31191656 Free PMC article.
References
-
- Babb SG, Matsudaira P, Sato M, Correia I, Lim S-S, Fimbrin in podosomes of monocyte-derived osteoclasts. Cell Motil.Cytosk. 37 (1997) 308–325. - PubMed
-
- Biswas R, Yuen D, Hruska K, MA. Chellaiah. Functional assessment of specific gelsolin peptide domains in osteoclast Actin Assembly and Motility. J Bone Miner Res 18 suppl1[Functional assessment of specific gelsolin peptide domains in osteoclast Actin Assembly and Motility], S279 10-10-2003.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

