Icariin Reduces Cartilage Degeneration in a Mouse Model of Osteoarthritis and is Associated with the Changes in Expression of Indian Hedgehog and Parathyroid Hormone-Related Protein
- PMID: 30244259
- PMCID: PMC6180903
- DOI: 10.12659/MSM.910983
Icariin Reduces Cartilage Degeneration in a Mouse Model of Osteoarthritis and is Associated with the Changes in Expression of Indian Hedgehog and Parathyroid Hormone-Related Protein
Abstract
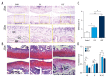
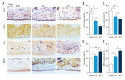
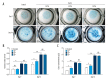
BACKGROUND The aim of this study was to determine the role of icariin, a Chinese traditional herbal medicine extracted from Epimedium, in osteoarthritis (OA), using the murine anterior cruciate ligament transection (ACLT)-induced model of OA and micromass culture of murine chondrocytes. MATERIAL AND METHODS Twenty-four three-month-old C57/6J mice were randomly divided into three groups: the sham group (no surgery and joint injection with normal saline) (N=8); the ACLT + ICA group (ACLT surgery and icariin treatment) (N=8); and the ACLT group (ACLT surgery and joint injection with normal saline) (N=8). At 12 weeks after ACLT surgery, murine articular cartilage was harvested from all mice for histological evaluation of any differences in cartilage degeneration. In vitro micromass culture of mouse chondrocytes was used to study the effects of icariin on chondrocyte differentiation and growth from the three mouse groups. RESULTS Icariin treatment (mice in the ACLT + ICA group) significantly reduced degeneration of cartilage in OA with increased cartilage thickness, associated with increased expression of collagen type II alpha 1 (COL2A1), decreased chondrocyte hypertrophy, and decreased expression of collagen type X (ColX) and matrix metalloproteinase 13 (MMP13). In vitro, icariin promoted chondrocyte differentiation by upregulating the expression of agrrecan, Sox9 and parathyroid hormone-related protein (PHrP) and down-regulation of Indian hedgehog (Ihh) and genes regulated by Ihh. CONCLUSIONS In a mouse model of OA icariin treatment reduced destruction of cartilage, promoted chondrocyte differentiation, upregulated expression of PHrP and down-regulated the expression of Ihh.
Conflict of interest statement
None.
Figures



References
-
- Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386:376–87. - PubMed
-
- Sinusas K. Osteoarthritis: Diagnosis and treatment. Am Fam Physician. 2012;85:49–56. - PubMed
-
- Hunziker EB, Lippuner K, Keel MJ, Shintani N. An educational review of cartilage repair: Precepts and practice, myths and misconceptions, progress and prospects. Osteoarthritis Cartilage. 2015;23:334–50. - PubMed
-
- Fosang AJ, Beier F. Emerging frontiers in cartilage and chondrocyte biology. Best Pract Res Clin Rheumatol. 2011;25:751–66. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

