Amyloid burden identifies neuropsychological phenotypes at increased risk of progression to Alzheimer's disease in mild cognitive impairment patients
- PMID: 30244387
- PMCID: PMC6333718
- DOI: 10.1007/s00259-018-4149-2
Amyloid burden identifies neuropsychological phenotypes at increased risk of progression to Alzheimer's disease in mild cognitive impairment patients
Abstract
Purpose: The extent of amyloid burden associated with cognitive impairment in amnestic mild cognitive impairment is unknown. The primary aim of the study was to determine the extent to which amyloid burden is associated to the cognitive impairment. The secondary objective was to test the relationship between amyloid accumulation and memory or cognitive impairment.
Materials and methods: In this prospective study 66 participants with amnestic mild cognitive impairment underwent clinical, neuropsychological and PET amyloid imaging tests. Composite scores assessing memory and non-memory domains were used to identify two clinical classes of neuropsychological phenotypes expressing different degree of cognitive impairment. Detection of amyloid status and definition of optimal amyloid ± cutoff for discrimination relied on unsupervised k-means clustering method.
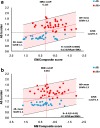
Results: Threshold for identifying low and high amyloid retention groups was of SUVr = 1.3. Aß + participants showed poorer global cognitive and episodic memory performance than subjects with low amyloid deposition. Aß positivity significantly identified individuals with episodic memory impairment with a sensitivity and specificity of 80 and 79%, (χ2 = 21.48; P < 0.00001). Positive and negative predictive values were 82 and 76%, respectively. Amyloid deposition increased linearly as function of memory impairment with a rate of 0.13/ point of composite memory score (R = -44, P = 0.0003).
Conclusion: The amyloid burden of SUVr = 1.3 allows early identification of subjects with episodic memory impairment which might predict progression from MCI to Alzheimer's disease.
Trial registration: EudraCT 2015-001184-39.
Keywords: Beta-amyloid; Cognitive trajectory; Memory performance; Mild cognitive impairment; PET imaging.
Conflict of interest statement
Conflict of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures

References
-
- AmericanPsychiatricAssociation . Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association; 2013.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

