Targeting the Extracellular Signal-Regulated Kinase 5 Pathway to Suppress Human Chronic Myeloid Leukemia Stem Cells
- PMID: 30245209
- PMCID: PMC6178886
- DOI: 10.1016/j.stemcr.2018.08.016
Targeting the Extracellular Signal-Regulated Kinase 5 Pathway to Suppress Human Chronic Myeloid Leukemia Stem Cells
Abstract
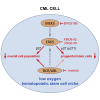
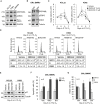
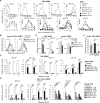
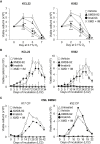
Tyrosine kinase inhibitors (TKi) are effective against chronic myeloid leukemia (CML), but their inefficacy on leukemia stem cells (LSCs) may lead to relapse. To identify new druggable targets alternative to BCR/ABL, we investigated the role of the MEK5/ERK5 pathway in LSC maintenance in low oxygen, a feature of bone marrow stem cell niches. We found that MEK5/ERK5 pathway inhibition reduced the growth of CML patient-derived cells and cell lines in vitro and the number of leukemic cells in vivo. Treatment in vitro of primary CML cells with MEK5/ERK5 inhibitors, but not TKi, strikingly reduced culture repopulation ability (CRA), serial colony formation ability, long-term culture-initiating cells (LTC-ICs), and CD26-expressing cells. Importantly, MEK5/ERK5 inhibition was effective on CML cells regardless of the presence or absence of imatinib, and did not reduce CRA or LTC-ICs of normal CD34+ cells. Thus, targeting MEK/ERK5 may represent an innovative therapeutic approach to suppress CML progenitor/stem cells.
Keywords: CML; ERK5/MAPK; MAP2K5; MAPK7; combination therapy; hypoxia; leukemia stem cells; microenvironment; stem cell niche; tyrosine kinase inhibitors/TKi.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Selective JAK2/ABL dual inhibition therapy effectively eliminates TKI-insensitive CML stem/progenitor cells.Oncotarget. 2014 Sep 30;5(18):8637-50. doi: 10.18632/oncotarget.2353. Oncotarget. 2014. PMID: 25226617 Free PMC article.
-
Overcoming BCR::ABL1 dependent and independent survival mechanisms in chronic myeloid leukaemia using a multi-kinase targeting approach.Cell Commun Signal. 2023 Nov 29;21(1):342. doi: 10.1186/s12964-023-01363-2. Cell Commun Signal. 2023. PMID: 38031192 Free PMC article.
-
BCR/ABL kinase inhibition by imatinib mesylate enhances MAP kinase activity in chronic myelogenous leukemia CD34+ cells.Blood. 2004 Apr 15;103(8):3167-74. doi: 10.1182/blood-2003-04-1271. Epub 2003 Dec 11. Blood. 2004. PMID: 15070699
-
Kinase-inhibitor-insensitive cancer stem cells in chronic myeloid leukemia.Expert Opin Biol Ther. 2014 Mar;14(3):287-99. doi: 10.1517/14712598.2014.867323. Epub 2014 Jan 3. Expert Opin Biol Ther. 2014. PMID: 24387320 Review.
-
Characterization of cancer stem cells in chronic myeloid leukaemia.Biochem Soc Trans. 2007 Nov;35(Pt 5):1347-51. doi: 10.1042/BST0351347. Biochem Soc Trans. 2007. PMID: 17956348 Review.
Cited by
-
Modulation of cell physiology under hypoxia in pancreatic cancer.World J Gastroenterol. 2021 Jul 28;27(28):4582-4602. doi: 10.3748/wjg.v27.i28.4582. World J Gastroenterol. 2021. PMID: 34366624 Free PMC article. Review.
-
In Vitro Comparison of the Effects of Imatinib and Ponatinib on Chronic Myeloid Leukemia Progenitor/Stem Cell Features.Target Oncol. 2020 Oct;15(5):659-671. doi: 10.1007/s11523-020-00741-x. Target Oncol. 2020. PMID: 32780298 Free PMC article.
-
Impact of ERK5 on the Hallmarks of Cancer.Int J Mol Sci. 2019 Mar 21;20(6):1426. doi: 10.3390/ijms20061426. Int J Mol Sci. 2019. PMID: 30901834 Free PMC article. Review.
-
Molecular Mechanisms of Epithelial to Mesenchymal Transition Regulated by ERK5 Signaling.Biomolecules. 2021 Jan 29;11(2):183. doi: 10.3390/biom11020183. Biomolecules. 2021. PMID: 33572742 Free PMC article. Review.
-
Targeting the RBM39-MEK5 axis synergizes with bortezomib to inhibit the malignant growth of multiple myeloma.Blood Adv. 2025 Apr 22;9(8):1991-2005. doi: 10.1182/bloodadvances.2025015815. Blood Adv. 2025. PMID: 40048740 Free PMC article.
References
-
- Blom T., Nilsson G., Sundström C., Nilsson K., Hellman L. Characterization of a human basophil-like cell line (LAMA-84) Scand. J. Immunol. 1996;44:54–61. - PubMed
-
- Carvajal-Vergara X., Tabera S., Montero J.C., Esparís-Ogando A., López-Pérez R., Mateo G., Gutiérrez N., Parmo-Cabañas M., Teixidó J., San Miguel J.F. Multifunctional role of Erk5 in multiple myeloma. Blood. 2005;105:4492–4499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

