Raging the War Against Inflammation With Natural Products
- PMID: 30245627
- PMCID: PMC6137277
- DOI: 10.3389/fphar.2018.00976
Raging the War Against Inflammation With Natural Products
Abstract
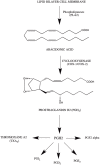
Over the last few decade Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) are the drugs of choice for treating numerous inflammatory diseases including rheumatoid arthritis. The NSAIDs produces anti-inflammatory activity via inhibiting cyclooxygenase enzyme, responsible for the conversation of arachidonic acid to prostaglandins. Likewise, cyclooxegenase-2 inhibitors (COX-2) selectively inhibit the COX-2 enzyme and produces significant anti-inflammatory, analgesic, and anti-pyretic activity without producing COX-1 associated gastrointestinal and renal side effects. In last two decades numerous selective COX-2 inhibitors (COXIBs) have been developed and approved for various inflammatory conditions. However, data from clinical trials have suggested that the prolong use of COX-2 inhibitors are also associated with life threatening cardiovascular side effects including ischemic heart failure and myocardial infection. In these scenario secondary metabolites from natural product offers a great hope for the development of novel anti-inflammatory compounds. Although majority of the natural product based compounds exhibit more selectively toward COX-1. However, the data suggest that slight structural modification can be helpful in developing COX-2 selective secondary metabolites with comparative efficacy and limited side effects. This review is an effort to highlight the secondary metabolites from terrestrial and marine source with significant COX-2 and COX-2 mediated PGE2 inhibitory activity, since it is anticipated that isolates with ability to inhibit COX-2 mediated PGE2 production would be useful in suppressing the inflammation and its classical sign and symptoms. Moreover, this review has highlighted the potential lead compounds including berberine, kaurenoic acid, α-cyperone, curcumin, and zedoarondiol for further development with the help of structure-activity relationship (SAR) studies and their current status.
Keywords: anti-inflammatory; cyclooxygenase pathway; cyclooxygenase-2; inflammation; natural products; prostaglandin E2.
Figures




Similar articles
-
Dual acting anti-inflammatory drugs: a reappraisal.Pharmacol Res. 2001 Dec;44(6):437-50. doi: 10.1006/phrs.2001.0872. Pharmacol Res. 2001. PMID: 11735348 Review.
-
Anti-inflammatory drugs in the 21st century.Subcell Biochem. 2007;42:3-27. doi: 10.1007/1-4020-5688-5_1. Subcell Biochem. 2007. PMID: 17612044 Review.
-
Cyclooxygenase inhibitory natural products: current status.Curr Med Chem. 2006;13(6):659-78. doi: 10.2174/092986706776055698. Curr Med Chem. 2006. PMID: 16529558 Review.
-
Synthetically-tailored and nature-derived dual COX-2/5-LOX inhibitors: Structural aspects and SAR.Eur J Med Chem. 2021 Dec 5;225:113804. doi: 10.1016/j.ejmech.2021.113804. Epub 2021 Aug 27. Eur J Med Chem. 2021. PMID: 34479036 Review.
-
Natural-Derived COX-2 Inhibitors as Anticancer Drugs: A Review of their Structural Diversity and Mechanism of Action.Anticancer Agents Med Chem. 2023;23(1):15-36. doi: 10.2174/1389450123666220516153915. Anticancer Agents Med Chem. 2023. PMID: 35638275 Review.
Cited by
-
Naturally Occurring Xanthones and Their Biological Implications.Molecules. 2024 Sep 6;29(17):4241. doi: 10.3390/molecules29174241. Molecules. 2024. PMID: 39275090 Free PMC article. Review.
-
Anti-Inflammatory Medicinal Plants of Bangladesh-A Pharmacological Evaluation.Front Pharmacol. 2022 Mar 24;13:809324. doi: 10.3389/fphar.2022.809324. eCollection 2022. Front Pharmacol. 2022. PMID: 35401207 Free PMC article. Review.
-
CLK2 mediates IκBα-independent early termination of NF-κB activation by inducing cytoplasmic redistribution and degradation.Nat Commun. 2024 May 9;15(1):3901. doi: 10.1038/s41467-024-48288-z. Nat Commun. 2024. PMID: 38724505 Free PMC article.
-
Levels of Cyclooxygenase 2, Interleukin-6, and Tumour Necrosis Factor-α in Fibroblast Cell Culture Models after Photobiomodulation at 660 nm.Oxid Med Cell Longev. 2021 Feb 13;2021:6667812. doi: 10.1155/2021/6667812. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33628374 Free PMC article.
-
The Role of the Nuclear Factor-Kappa B (NF-κB) Pathway in SARS-CoV-2 Infection.Pathogens. 2024 Feb 12;13(2):164. doi: 10.3390/pathogens13020164. Pathogens. 2024. PMID: 38392902 Free PMC article. Review.
References
-
- Aggarwal S., Ichikawa H., Takada Y., Sandur S. K., Shishodia S., Aggarwal B. B. (2006). Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IκBα kinase and Akt activation. Mol. Pharmacol. 69, 195–206. 10.1124/mol.105.017400 - DOI - PubMed
-
- Ahn K. S., Noh E. J., Zhao H. L., Jung S. H., Kang S. S., Kim Y. S. (2005). Inhibition of inducible nitric oxide synthase and cyclooxygenase II by Platycodon grandiflorum saponins via suppression of nuclear factor-kappaB activation in RAW 264.7 cells. Life Sci. 76, 2315–2328. 10.1016/j.lfs.2004.10.042 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

