Driving Ability in Alzheimer Disease Spectrum: Neural Basis, Assessment, and Potential Use of Optic Flow Event-Related Potentials
- PMID: 30245666
- PMCID: PMC6137098
- DOI: 10.3389/fneur.2018.00750
Driving Ability in Alzheimer Disease Spectrum: Neural Basis, Assessment, and Potential Use of Optic Flow Event-Related Potentials
Abstract
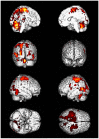
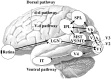
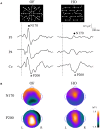
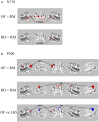
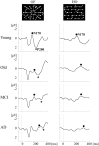
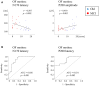
Driving requires multiple cognitive functions including visuospatial perception and recruits widespread brain networks. Recently, traffic accidents in dementia, particularly in Alzheimer disease spectrum (ADS), have increased and become an urgent social problem. Therefore, it is necessary to develop the objective and reliable biomarkers for driving ability in patients with ADS. Interestingly, even in the early stage of the disease, patients with ADS are characterized by the impairment of visuospatial function such as radial optic flow (OF) perception related to self-motion perception. For the last decade, we have studied the feasibility of event-related potentials (ERPs) in response to radial OF in ADS and proposed that OF-ERPs provided an additional information on the alteration of visuospatial perception in ADS (1, 2). Hence, we hypothesized that OF-ERPs can be a possible predictive biomarker of driving ability in ADS. In this review, the recent concept of neural substrates of driving in healthy humans are firstly outlined. Second, we mention the alterations of driving performance and its brain network in ADS. Third, the current status of assessment tools for driving ability is stated. Fourth, we describe ERP studies related to driving ability in ADS. Further, the neural basis of OF processing and OF-ERPs in healthy humans are mentioned. Finally, the application of OF-ERPs to ADS is described. The aim of this review was to introduce the potential use of OF-ERPs for assessment of driving ability in ADS.
Keywords: Alzheimer disease spectrum; Alzheimer's disease; driving ability; event-related potentials; mild cognitive impairment; radial optic flow perception.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

